《1.Introduction》
1.Introduction
Soybean (Glycine max(L.) Merr.) is considered to be one of the oldest crops cultivated by humans[1]. It is believed to have originated in China, probably in the northern and central regions[2]. Some evidence indicates that soybean was domesticated as early as 3500 BCE[3], and was subsequently introduced into Korea around 200 BCE and into Japan and Russia around 300 CE[1]. Annual world production of soybean is approximately 104.5 billion USD.
In 1899, damage to soybean from soybean cyst nematode (SCN, Heterodera glycines) was described in China as "fire-burned seedlings”[3]. Since the plant originated in China, and as the nematode caused "fire-burned seedling”disease in that country before much dissemination of the cultivated crop had occurred, China is believed to be the origin of the nematode. SCN continues to affect soybean production in China, with yield losses estimated at more than 120 million USD[4–7], and is now reported in many countries where soybean is produced. SCN was first observed in 1915 in Japan[8] and later in the United States[9], where it causes annual yield losses of more than 1.2 billion USD[9–11]. SCN is now present throughout South America.
The most common sources of resistance to SCN that are used in crop rotation in the northern central United States include PI 88788, PI 54840 (Peking), and PI 437654[12,13]. Unfortunately, due to the continuous use of these resistant sources, more virulent forms of SCN have evolved over time. The virulence phenotypes of SCN populations have been described as race based on four soybean genotypes[14]. A system of HG Type (HG represents the first letters of the genus and species names of the nematode, Heterodera glycines) was subsequently developed in order to determine the virulence of SCN populations; the system used seven soybean indicator lines with various forms of resistance because race is inap-propriate for characterizing diverse, heterogeneous populations of SCN[15]. There is an increasing evidence of SCN populations that are able to overcome resistance[16–18]. Hence, the need to diversify the resistant sources or broaden the resistance background in elite soybean germplasm is increasing, as more SCN populations adapt to break the resistance in PI 88788[19–23].
《2.Molecular adaptation to obligate parasitism》
2.Molecular adaptation to obligate parasitism
Heterodera glycines, like other cyst nematodes, is an obligate root parasite that has complex and intimate interactions with its hosts. The motile, second-stage juvenile (J2) in soil invades the root of soybean plants using its stylet. The J2 then moves intracellularly through the root cortical cells toward the stele,where an initial cell is induced to form a syncytium through the secretions from the esophageal gland cells. Feeding commences,and after three successive molts, the J2 becomes an adult. The syncytium is a meta-bolic sink that serves as a feeding site and provides the nutrients required for development into adulthood[24]. The nematode completely depends on the syncytium for its survival, which means that the destruction or death of the syncytial cell will result in the death of the nematode.
Molecular studies have established that secreted proteins from the esophageal gland cells of the nematode are crucial to this intimate relationship[25,26]. Virulence genes (ror) from SCN that enable its reproduction on resistant soybean cultivars were reported in a classical genetic study[27]. Meanwhile, a number of cellulase and pectate lyases[28–32] have been characterized in SCN before and after infection of soybean roots. These cellulases probably function to soften root tissues, since they are present in infective juveniles that are invading root tissues and in males that need to exsheath the third stage cuticle and exit the roots[30]; other genes from SCN,such as guanylyl genes, may function in chemosensory recognition[33].
A chorismate mutase (CM) gene from SCN showing polymorphism has been characterized[34]. This enzyme is found in the shikimate pathway of plants and does not exist in animals. It could alter one or more of the downstream products of the shikimate pathway that may perform a role in syncytia maintenance. A secreted CLAVATA3/ Embryo surrounding region-related (CLE) peptide homologous to the CLAVATA3 ligand, with signal peptides that induce cell division in plants (protoplasts), was present in this nematode[35]. Recently, a CLE peptide from SCN (HgCLE) was found to interact with soybean CLE receptors in an in vitro study, and silencing of the receptors increased resistance to SCN[36].
Other genes recently identified in SCN include a biotin synthase and a putative soluble N-ethylmaleimide-sensitive factor activating protein receptor (SNARE) domain gene[37]. A SNARE-bearing protein HgSLP-1 could interact with the soybean N-ethylmaleimide-sensitive factor attachment proteinα (α-SNAP) to trigger defense response in an incompatible interaction. HgSLP-1 seems to be absent,suggesting its role as an avirulent SCN protein. Thus, interaction between HgSLP-1 from an avirulent SCN and Rhg1α-SNAP in soybean triggers a resistance response[37]. Although biotin is involved in several cellular processes in plants, the question of what the nematode biotin is doing in the plant is yet to be determined. However, it is speculated that amino acid differences in biotin between avirulent and virulent SCN may help in ascertaining its function[37]. In addition, three novel ran-binding protein genes containing signal peptides at the N-terminal and B30.2 and spla kinase and ryanodine receptor (SPRY) domains at the C-terminal have been cloned and characterized from Heterodera glycines. RNAi-mediated silencing of Hg-rbp-2 resulted in suppression and parasitic ability of the nematode[38].
It is clear that the molecular details of SCN parasitism on soybean and the mechanism of soybean resistance are not well understood; however, such knowledge is critical in finding novel strategies to engineer resistance.
《3.Compatible interactions》
3.Compatible interactions
When the nematode is able to successfully infest and reproduce on its host, the interaction is termed to be compatible. In this case,the infective J2 is able to invade the soybean plant root, penetrate and migrate through the root epidermis and cortical cells, and continue to the stele, where syncytial cell induction, development, and maintenance occur. Penetration and migration through the epidermis and cortical cells are both mechanical[39] and enzymatic[29]. Secretions from the amphids, gland cells, and inner labial sensilla form a short tube between the stylet and the syncytium[40–42]. The stylet protrudes in and out of the intact feeding plug during feeding[26]. Consequently,the soybean responds by gene expressional modifications and cellular changes, especially within the affected cells[43,44].
At first, the cytoplasm of the syncytial cell becomes dense,accompanied by increased ribosome and rough endoplasmic reticulum(RER)numbers[42]. The cell wall gradually dissolves through openings in affected cell walls. Plasmodesmata of the initial cell expand, leading to the fusion of protoplasts of contiguous cells and, ultimately, to the dissolution of cell walls, resulting in a multinucleate feeding cell (syncytium) with increased metabolic activity. The cytoplasm becomes dense with increased organelles and cell wall ingrowths[45,46]. There is thickening of the cell wall, and the main cell vacuole is replaced with many secondary vacuoles[42]. Cell wall dissolution is greater in cells that are more distant from the initial syncytial cell, whereas cell wall thickening is more extensive in cells that are closer to the initial syncytial cell[47]. Parenchyma cells may undergo hyperplasia,whereas cells distal to the syncytium undergo hypertrophy. It is not known whether the nuclei within the syncytium ever replicate. The nematode then feeds and molts three more times before becoming an adult (Fig.1(a) and(b)). The female lays eggs, which are fertilized by the males in order to begin a new generation of compatible relationship.
《4.Incompatible interactions》
4.Incompatible interactions
In incompatible interactions, the J2 invades and penetrates into the root and induces the formation of the syncytium; however, shortly after establishment, the syncytium becomes necrotic and degenerated, leading to the death of the nematode ( Fig.1(c) and (d)). The length of time it takes for necrosis formation and, ultimately, syncytium death depends on the host plant[48]. The nuclear degeneration and necrosis are the results of a hypersensitive reaction (Fig.1(e))[45,48,49]. Resistance response rapidity varies in different sources of resistance: Rapid syncytium degeneration was observed in Peking, whereas in PI 88788 and PI 209332, syncytial death is slow[48,50–52]. Most syncytia initiated in the soybean cultivar Peking stop developing and become necrotic within 5 days after inoculation (DAI)[53], probably beginning with dilation of the RER at as early as 2 DAI[52]. By 5 DAI, cell wall appositions form alongside necrosis of the developing syncytium[50,54]. Also, by 4–5 days after initiation,the following cellular changes occur: development of irregular thickenings of syncytial cell walls with appositions, and development of cell wall ingrowths and invagination of plasmalemma with numerous microtubules in the cytoplasm[41,42]. Development of irregular cell wall thickenings, cell wall appositions, cell necrosis, and degeneration of nuclei were observed in PI 437654, in a resistance response similar to that of Peking[55].
《Fig. 1》
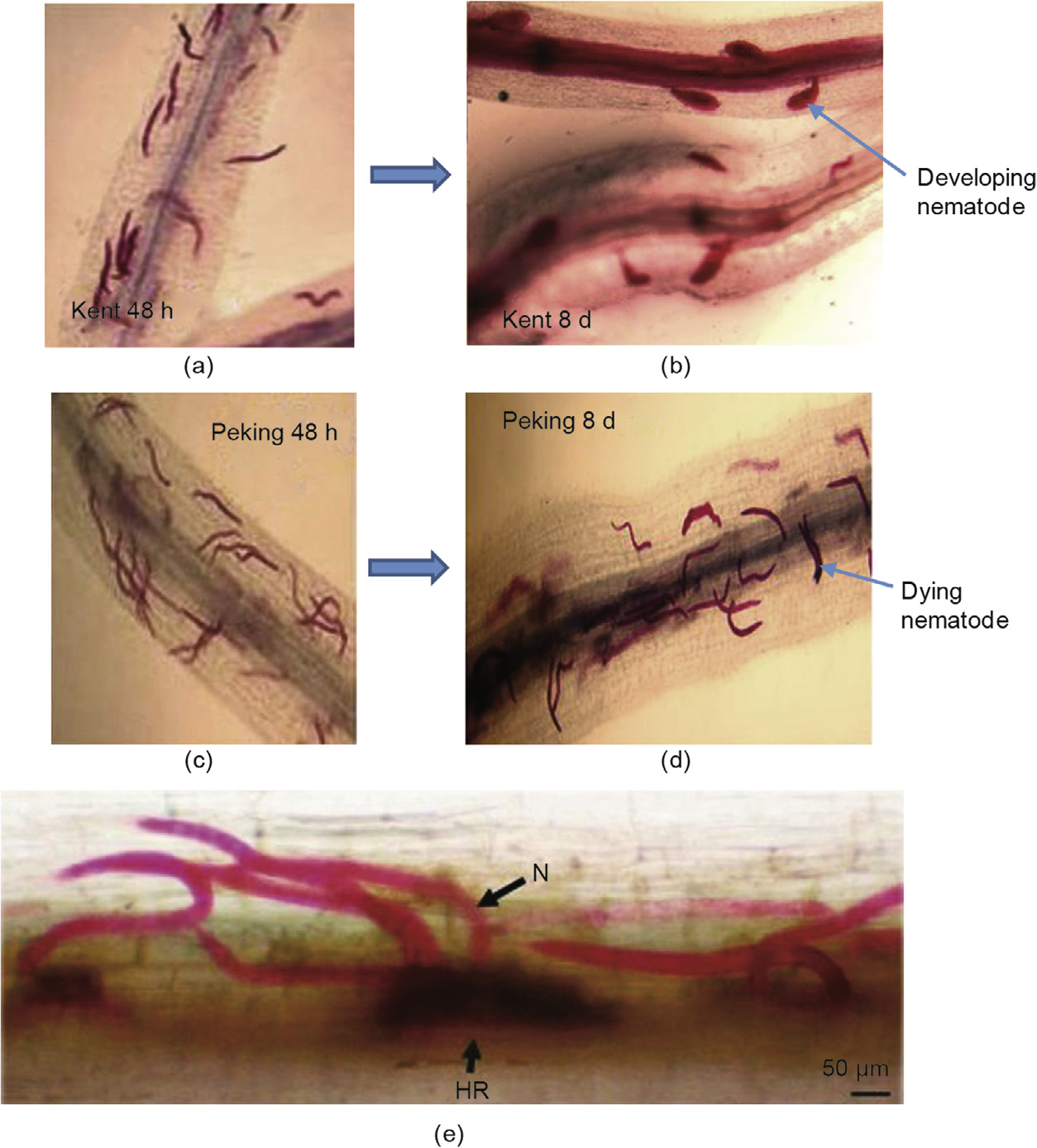
Fig.1.Compatible and incompatible interactions between soybean and SCN, Heterodera glycines. (a)J2 inside a susceptible host (Kent) root 48 h after inoculation and (b)8 days after inoculation(DAI); (c)J2 inside a resistant host (Peking) root 48 h after inoculation and (d)8 DAI; (e)image of a root of the resistant soybean cultivar Forrest showing hypersensitive response (HR)-like cell death at the site of feeding by infective SCN J2, which are stained pink with acid fuchsin(N). (Images(a–d) are courtesy of B.Mathews, United States Department of Agriculture; image(e)is courtesy of Xiaohong Liu, University of Missouri)
The initial syncytial cells produced in soybean cultivars from PI 88788 or in cultivars derived from these sources have extensive accumulation of cisternae and RER[42] without thickened cell walls, appositions, or a necrotic layer, which typically occur with the resistance responses in Peking[42]. Intriguingly, the cell walls of the non-syncytial cells surrounding the developing syncytium become necrotic,as does the entire syncytium by 8–10 DAI[50,54]. This is followed by nuclear degeneration and the formation of chromatin-like materials within the syncytial cell cytoplasm.
In general, the demise of syncytia in an incompatible interaction may be attributed to nuclei degeneration, cell wall apposition formation, non-functional endoplasmic reticulum,and programmed cell death[53]. However, gene expression changes in degenerating syncytia in PI 88788 and Peking suggest a genotype-specific gene expression,albeit with a conserved transcriptional background[56], which implies that the mechanism of SCN resistance in soybean may be related, yet distinct, in different cultivars.
《5.SCN-resistance genes described in soybean》
5.SCN-resistance genes described in soybean
Classical genetic studies have revealed that soybean resistance to SCN is conditioned by both the recessive genes designated as rhg1, rhg2, and rhg3[57] and the dominant genes designated as Rhg4 and Rhg5[58,59]. Subsequent studies suggest that this interaction is more complicated and involves both major and minor genes[60]. Advancement in genetic marker technology in the last two decades has tremendously aided the identification, localization, and characterization of major quantitative trait loci (QTL) controlling soybean resistance to SCN[16,61–64]. The results of many QTL experiments have shown that the genetic regions of Rhg1 and Rhg4 contribute most of the SCN resistance (Fig. 2)[65], so the molecular markers linking these regions were identified in order to select these loci in soybean lines[63,66,67].
《Fig. 2》
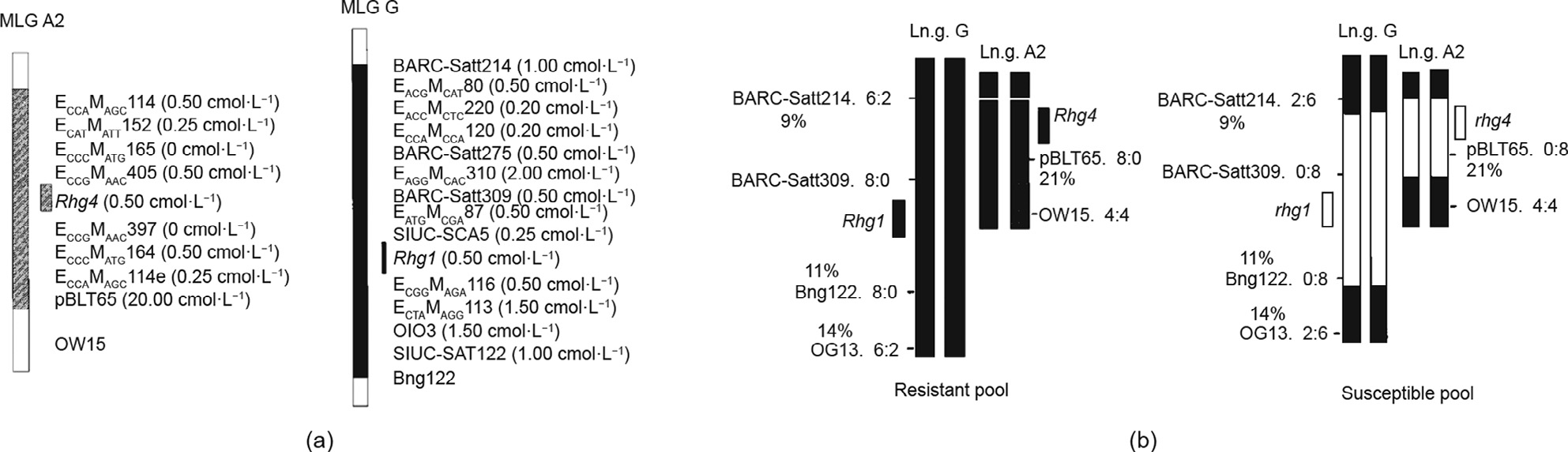
Fig.2.(a)High-density genetic map of the chromosomal segments carrying the Rhg1 and Rhg4 loci, and(b)their presence or absence in resistant and susceptible soybean (Glycine max) pools. MLG: molecular linkage group; Ln.g.:linkage group. (Adapted from Ref.[65])
The Rhg1 gene mapped onto soybean chromosome 18 shows incomplete dominance. This gene plays a significant role in SCN resistance[16]. Different Rhg1 alleles exist among different resistant sources[68]. About 90% of SCN-resistant sources in the United States use the rhg1-b allele. Rhg4,on the other hand, is mapped onto chromosome 8 and is dominant,and may be required for complete resistance in some resistant sources[68].
To further refine the Rhg1 and Rhg4 loci and to characterize candidate genes involved in SCN–soybean interaction, integrated approaches such as functional genomics tools,soybean genome sequencing, map-based cloning, mutagenesis, targeting induced local lesions in genomes (TILLING), and gene-silencing technologies have aided very intriguing discoveries in the last decade[51,69–74]. In particular, the discovery that a portion of the rhg1-b gene encodes three proteins—namely, an amino acid transporter (AAT), an a-SNAP, and a wound-inducible domain protein(WI12) —that contribute to SCN resistance in soybean was ground-breaking (Fig.3)[75]. A single copy of this 31 kb portion of rhg1-b is found in susceptible cultivars,but multiple copies are found in resistant cultivars(Fig.3).An increased number of copies of this segment results in increased expression of the gene in resistant cultivars.In addition,overexpression of these genes in susceptible cultivars produced some level of SCN suppression[75]. It is important to note that this result suggests that SCN resistance in soybean is conditioned not only by the presence or absence of the resistance locus Rhg1, but also by the copy number variation in a repeated 31 kb mult-gene segment at the rhg1-b locus.Other studies have also shown that the role of Rhg1 and Rhg4 in SCN resistance is independent of the leucine-rich repeat receptor-like kinase (LRR-RLK) associated with their QTL loci[74,76–78]. Interestingly, none of the genes in the repeat in the 31 kb multi-gene segment bears a resemblance to a classical plant resistance gene with a nucleotide-binding site–leucine-rich repeat (NBS-LRR) domain, suggesting a novel form of resistance in plants[76,78].
《Fig. 3》
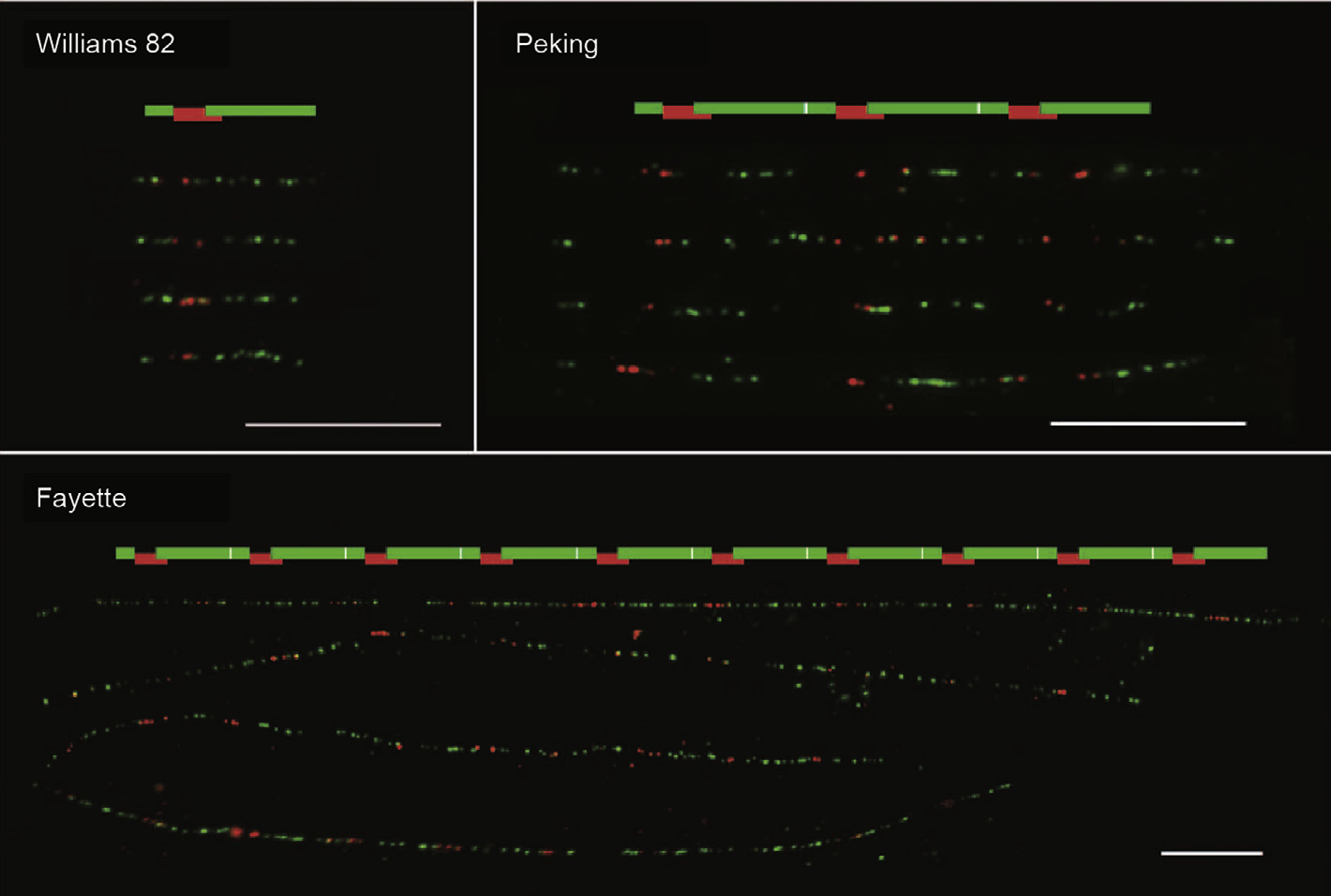
Fig.3. Fiber-fluorescence in situ hybridization (FISH) detection of Rhg1 copy number variation in widely used soybean lines: Probe diagram and composite of four fiber-FISH images (four DNA fibers) per genotype, revealing 10 or 3 direct repeat copies of the 31 kb Rhg1 segment in SCN-resistant Fayette and Peking, and 1 copy per Rhg1 haplotype in SCN-susceptible Williams 82. The white bars stand for 10 mm, which corresponds to approximately 32 kb using a 3.21 kb·mm-1 conversion rate. (Adapted from Ref.[75])
Current studies have confirmed that copy number variation, differences in gene sequence, and differences in methylation of the repeat segment mediate SCN resistance in soybean[79–81]. Based on these discoveries, soybean resistance has been grouped into high and low copy number accessions that have varying numbers of copies of the 31 kb portion of Rhg1[23,79]. Theα-SNAP allele in susceptible cultivars was reported to be different from the α-SNAP alleles in resistant cultivars in the C-terminal domain[79,80]. In addition, three different forms of α-SNAP proteins were observed among resistant lines with varying numbers of repeats, suggesting that the copy number and sequence of the α-SNAP protein in the Rhg1 play active roles in soybean resistance to SCN[80]. While multiple copies of α-SNAP from PI 88788-type resistance (GmSNAP18) simultaneously contribute to the rhg1-b resistance[75], the Peking-type GmSNAP18 alone at rhg1-a, together with Rhg4 , confers resistance to SCN at the locus[23]. Recently, genetic analysis revealed that GmSNAP11 serves as a novel minor resistance gene that contributes to an additive resistance against SCN[82].
Furthermore, a map-based cloning experiment showed that the Rhg4 locus contributing to resistance in Peking encodes a predicted cytosolic serine hydroxymethyltransferase (SHMT) that differs from the susceptible form in sequence[74]. Missense mutations that convert arginine (R) to proline (P) and tyrosine (Y) to asparagine (N) within the vitamin B6 binding sites of SHMT may be critical for its function in SCN resistance. These amino acid poly-morphisms may be responsible for the discrepancies in enzyme activity between the different forms of SHMT[74,83,84]. Recent findings indicate that SCN-resistant accessions that contain a low copy of Rhg1 require Rhg4 for resistance[80]; however, it is not known how the two loci interact to confer resistance at the molecular level[23]. Rhg1 in Peking requires simultaneous expression of Rhg4 in order to be functional, suggesting that epistasis may be involved in Peking-derived SCN resistance[68,74]. It has been shown that rhg1-a Peking-type GmSNAP18 is sufficient for resistance to SCN in combination with Rhg4, which reiterates the functional divergence of Peking-type GmSNAP18 in SCN resistance from that of GmSNAP18 in PI 88788[85]. Mutations on the SHMT revealed key residues for structural stability,ligand binding, enzyme activity,and protein interactions, thus providing compelling genetic evidence that SHMT is essential in conferring effective SCN resistance in Peking-type resistant cultivars, irrespective of whether a resistant Rhg1 allele is carried.
《6.Understanding the mechanism of SCN resistance in soybean》
6.Understanding the mechanism of SCN resistance in soybean
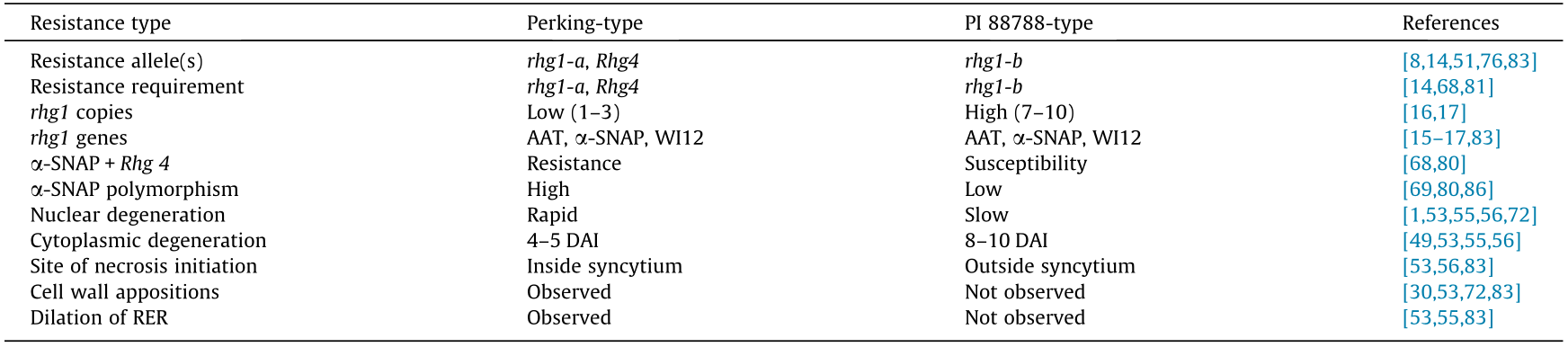
Even though several genetic, cytological,and molecular-mapping studies have aided the identification and characterization of genes contributing to SCN resistance in soybean, there is still a great deal to learn regarding the molecular mechanism underpinning SCN resistance. At present, two types of SCN resistance in soybean have been established,namely, PI 88788-type resistance and Perking-type resistance. Both types of resistance are mediated by two major QTL loci carrying the genes Rhg1 and Rhg4. SCN resistance in PI 88788 requires only the rhg1-b allele to be functional;however, resistance in Peking requires both the rhg1-a and Rhg4 alleles [16,23,85,65]. It is also known that in Peking-type resistance,there is a rapid and potent localized hypersensitive response that affects SCN J2, whereas in PI 88788-type resistance, the resistance response is more prolonged, and affects the SCN third-and fourth-stage juvenile. A comparison of the two types of SCN resistance in soybean is provided in Table 1 [1, 8, 14–17, 30, 49, 51, 53, 55, 56, 68, 69, 72, 76, 80, 81, 83, 86].
《Table 1》
Table 1 A comparison of the two types of SCN resistance in soybean.
The conundrum that remains to be resolved is the role, if any, played by rhg1 and rhg4 alleles in the up-or down-regulation of several genes during SCN–soybean incompatible interactions[56,87–93]. Biosynthetic pathways of several chemical products, such as jasmonic acid and phenylpropanoid, adenosylmethionine, ethylene, and so forth, were involved in the resistance response[94]. In spite of a network of molecular events during SCN–soybean incompatible interaction, there is a conserved differential gene expression in Peking and PI 88788, suggesting that there are particular genes involved in SCN resistance in Glycine max[56]. Up-regulation of salicylic acid (SA) pathway-related genes during the resistance response has been reported[56,89,95,96]. These molecular events eventually contribute to syncytia degeneration in an incompatible interaction. These defense-related genes are up-regulated within syncytia as a direct resistance response[23,89].
The activity of S-adenosyl-L-methionine(SAM)-dependent salicylic acid carboxyl methyltransferase 1 (GmSAMT1) in soybean resistance response to SCN has been reported[96]. When GmSAMT1 was overexpressed in different susceptible soybean lines, resistance to SCN was enhanced [97,98]. However, the interaction between SA and Rhg1 or Rhg4 during the resistance response is unknown. In another incompatible interaction, the polygalacturonase level was reduced when Peking and PI 88788 were infected by SCN[99], whereas levels of an ethylene-related protein (GmEREBP1) increased in infected soybean roots by the third day and sixth day in the resistant PI 437654, but decreased after six days in a susceptible cultivar[100]. Similarly, in an incompatible reaction with the cultivar Centennial (Peking source of resistance), phytoalexin glyceollin I increased 8 h after penetration by SCN J2, and increased to a maximum concentration of 23µg.g-1 of roots at 6 d after penetration[101]; in contrast,6 d after infection, the glyceollin I concentration was 7µg.g-1 of roots during a compatible interaction.In addition, other proteins such as 4-coumaroyl CoA ligase and phenylalanine ammonialyase increased in the incompatible host compared with in the compatible host, and showed greater activity in the cultivar Hartwig than in the cultivar Forest, which has a Peking source of resistance[102].
Another soybean gene,GmDS1, which encodes a receptor-like membrane protein(7.9 kDa), induced pathogen-and pest-associated molecular pattern (PAMP)-triggered immunity against multiple pests such as SCN and a fungal pathogen[103]. It is evident that the SCN–soybean interaction is complex, and is controlled by multi-genes at the Rhg1 and Rhg4 loci, whose expression is dependent on the copy number and on nucleotide variations, methylation, and the epistatic relationship between these three factors, with the involvement of other minor genes. Our understanding of the function of these genes and of how they interact or how methylation patterns affect SCN–soybean interaction will be critical for breeding SCN-resistant cultivars.
《7.Marker-assisted selection of SCN resistance》
7.Marker-assisted selection of SCN resistance
Plant breeders traditionally select SCN-resistant lines based on the response of the lines to SCN infection under greenhouse and field conditions[16]. This process is not only difficult but also labor, time, and capital intensive, and it is made even more complex by genetic variability in SCN populations[16]. Marker-assisted selection (MAS) is, however, based on alleles at genetic markers that are linked to SCN-resistance genes of interest[104]. The estimated cost of genotyping per data point using simple sequence repeat (SSR) markers is 0.25–1.00 USD, and requires 1–2 d; in contrast, using a greenhouse bioassay costs 1.50–5.00 USD per data point and requires 30 d[16].
Molecular markers have been identified in order to facilitate soybean breeding programs, including restriction fragment length polymorphisms (RFLPs), amplified fragment length polymorphism (AFLP), random amplified polymorphic DNA (RAPD), sequence-characterized amplified region (SCAR), and SSR markers and single nucleotide polymorphisms (SNPs) [16, 66, 67, 77, 86, 105 –109]. In addition to traditional QTL mapping using populations derived from bi-parental crosses, genome-wide association study (GWAS) using diverse and naturally occurring populations has been employed as an effective strategy for identifying QTL and elucidating the genetic basis of cyst nematode resistance in soybean[107–111]. Plant GWAS is becoming popular mainly due to advances in genome-sequencing technologies and to the capacity to generate more precise QTL positions with a sufficient number of genetic markers that can be identified[109]. Functional SNP markers to select either the rhg1 resistance allele or the Rhg4 resistance allele and to differentiate between PI 88788-and Peking-type resistances have been developed for high-throughput MAS[111,112]. At present,molecular assays are available to predict the Rhg1 copy number in soybean resistant lines[80,113,114], which will help to improve resistance selection and breeding accuracy.
《8.Implications for SCN-resistance breeding》
8.Implications for SCN-resistance breeding
The major loci, Rhg1 and Rhg4, controlling SCN resistance in soybean seem to have different evolutionary origins. The Rhg4 locus encoding the enzyme SHMT is absent in wild-type soybean cultivars, whereas the sensitive allele,rhg4, is present in wild-type soybean cultivars[83]. The Rhg4 locus contains two critical missense mutations that are absent in the sensitive allele, rhg4, suggesting that the Rhg4 allele involved in SCN resistance may have arisen through selection during domestication[74,83,84]. The implication is that artificial, directed missense mutations can be produced within the Rhg4 locus to create SHMT isoforms with increased enzymatic activity in new breeding lines.
The evolutionary scenario for the Rhg1 gene seems to be quite different from that of Rhg4. The Rhg1 gene locus is present in both the wild-type soybean, Glycine soja,and the domesticated type, Glycine max,intimating that the origin of Rhg1 likely predates domestication and divergence[80]. Soybean lines PI 468916 from Glycine soja and PI 438489B or PI 89772 from Glycine max are resistant to several SCN populations and carry novel genes for resistance[115,116]. Likewise, other SCN-resistant lines do not carry the common resistance Rhg1 and Rhg4 loci[111]. These findings suggest the prospect of identifying novel resistance genes that—once successfully identified, characterized, and introgressed into soybean—would provide alternative genes for resistance to SCN.
Glycine soja contains three copies of the 31.2 kb tandem repeat units at Rhg1, whereas some of the Glycine max accessions can have up to 10 copies of the tandem repeat units[75] .It has been suggested that the repeat originally came as a duplication and recombination[80]. This gene-duplication event probably accounts for the existence of more repeats in the cultivated SCN-resistant accessions.
Further enhancement of resistance can be accomplished by pyramiding genes from a variety of resistant sources, which may lead to an increase in the copy numbers of the resistance alleles. Gene stacking of the Rhg4 and Rhg1 alleles with increased copies of the Rhg1 allele may broaden resistance to SCN. Combining SCN-resistance alleles from different sources through backcrossing studies revealed that stacking genes from different resistant sources may broaden the SCN-resistance background[117]. In addition , resistance can be enhanced by the overexpression of plant defense-related genes. It is interesting to note that other SCN-resistant QTL that differ from Rhg1 and Rhg4 have been identified in Glycine soja[118]. When the resistant alleles in Glycine soja were stacked with Rhg1 and Rhg4 alleles, resistance against SCN was increased[119].
Recent genetic manipulation studies show that the silencing or overexpression of certain genes in soybean plants can lead to increased SCN resistance[36,97]. In a parallel study, knocking down a ran-binding protein gene (Hg-rbp-2) from SCN reduced SCN root invasion and development[38]. While many experiments indicate that it is feasible to achieve SCN resistance in soybean by means of the genetic manipulation of genes other than Rhg1 and Rhg4, much more work lies ahead in our attempts to improve breeding efficiency and scale up the production of transgenic SCN-resistant soybean lines for the use of farmers.
《Acknowledgements》
Acknowledgements
The authors express their sincere appreciation to the North Dakota Soybean Council,USA, for their funding support for the soybean cyst nematode research program.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Guiping Yan and Richard Baidoo declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




