《1.Wheat evolution and synthetic hexaploid wheat》
1.Wheat evolution and synthetic hexaploid wheat
《1.1.Wheat evolution and domestication》
1.1.Wheat evolution and domestication
Common wheat (Triticum aestivum L.(T.aestivum L.)) arose through natural hybridization and chromosome doubling between T.turgidum ssp.durum Desf. MacKey, a cultivated allotetraploid (2n=4x=28,AABB), and Aegilops tauschii Coss. (Ae.tauschii Coss.), a diploid goat grass (2n=4x=14,DD) [1–4]. Since its arrival,common wheat has become a popular staple crop, not only because it adapts well to different photoperiod and vernalization conditions, but also because it grows more robustly than its progenitors under salt, low pH, aluminum, and frost conditions. In addition, it has enhanced resistance to pathogens as well as versatile end products [5].
Common wheat provides a good model to characterize structural, functional, and epigenetic changes in an allopolyploid genome. Studying the unique features of wheat allohexaploids and comparing them with those in other allopolyploid plants will reveal new insights into the molecular mechanisms for wheat’s success. It is not surprising that two rounds of allopolyploidization make the genome of common wheat extremely complex; however, they also provide a rare genomic system for studying the rules of homeolog interaction that may underlie unique traits of wheat as a successful polyploid plant.
Synthetic hexaploid wheat (SHW) is an artificially created hex-aploid wheat that represents a wider genetic basis because of the introduction of additional genetic resources from tetraploid and diploid relatives of wheat. With no significant reproduction barrier, SHW lines are useful in introducing agronomically needed traits into common wheat from wild genetic resources.
《1.2.Why do we need synthetic wheat?》
1.2.Why do we need synthetic wheat?
In order to continue feeding the world’s increasing population, it will be necessary in the next 50 years to grow more wheat than the total amount of wheat that was produced over the past 10 000 years. In the past half century, the yield potential of common wheat varieties has been increasing. Recently, however, this trend is slowing down ,causing concerns about the world’s future food security.For example, over the past 12 years, the national average wheat yield of the UK has remained at around 8 t · hm-2, without further increments [6]. The erosion of wheat diversity should be blamed for such a slowdown of wheat yield per hectare. Limited genetic diversity can be explored within the current germplasm pool. It is high time to turn to the wild relatives of wheat, which have been explored on and off with no systematic effort. Repeating the ancient hybridization between goat grass and emmer or durum wheat via SHW should enable wheat researchers to introduce novel genetic sources, since many valuable genetic variations in the wild relatives were lost during wheat origination and domestic ation. Free crossing between SHW and modern wheat varieties should introduce the necessary new traits to improve both yield and stress tolerance in wheat.
《1.3.How are SHWs produced?》
1.3.How are SHWs produced?
Most SHW lines have been developed by crossing durum (pasta) wheat (T.turgidum ssp.durum, AABB) and wild goat grass (Ae.tauschii,DD). In most cases, the diploid species is used as a paternal parent to pollinate durum wheat. The reciprocal cross is possible, albeit with less success due to smaller embryo sizes or embryo defects. In certain cases, embryos derived from the interspecific cross (durum Ae. tauschii) may develop, but endosperms may not. Thus, it is necessary to conduct embryo rescue 2–3 weeks after pollination. During this process, embryos are dissected from immature seeds and transferred to an agar medium with nutrients such as sugar and salt for proper development. The type of the tetraploid parent, such as a different accession of durum wheat, may affect endosperm formation in the cross. For example, Langdon is a well-known durum variety that has a relatively high endosperm development rate. This durum variety is preferred for synthetic wheat production so as to skip the embryo rescue process. The regenerated plants are triploid, and are usually treated with colchicine to double their chromosome number before they are trans-planted to soil in pots to produce mature plants and, ultimately, seeds. In addition to the conventional artificial colchicine treatment, chromosome doubling can be achieved by the union of unreduced gametes (2n) derived from spontaneous meiotic restitution in T. turgidum–Ae.tauschii hybrids [7,8]. In fact,spontaneous chromosome doubling has been widely observed during the creation of new SHW lines [7–10]. These SHW lines are useful for producing amphidiploids and double haploids for genetic improvement of existing wheat varieties [11].
《2.An overview of SHW history》
2.An overview of SHW history
The first attempt to develop synthetic wheat was made in the middle of the last century with "synthetic spelta”in a study to determine the progenitors of T.aestivum subsp.spelta L.Thell [4]. These earliest allopolyploid hybrid forms of common wheat were named "synthetic hexaploid wheat.”Since the late 1980s, the International Maize and Wheat Improvement Center (CIMMYT) has developed more than 1000 SHW lines [12]. In subsequent studies, SHW has been recognized and confirmed as a valuable genetic source with better performance under biotic and abiotic stresses, as well as with better yield potential such as larger kernels and spikes [13,14]. However, synthetic wheat itself cannot be used as a cultivar because of the presence of "wild characters,”or agronomically undesirable characters such as tenacious glumes that causes non-free threshing grains; it is necessary to remove these characters or transfer desired traits of synthetic wheat into common wheat varieties by developing synthetic derivative lines (SDLs) through crossing with elite common wheat cultivars [6]. In 2003, Spain pre-registered a CIMMYT synthetic wheat derivative under the name Carmona [15]. At the same time, China also registered the first synthetic-derived cultivar. Since then, at least 62 SDLs have been registered as cultivars around the world (Table 1). Synthetic derivatives showed a significant increase in genetic diversity when compared with their parents [16].
About a quarter of the world’s population suffers from health issues caused by iron (Fe) deficiency[17]. Regular wheat grains do not provide sufficient Fe and zinc (Zn) for people who rely on wheat as a main supply. SHW has been regarded as an important source for developing micronutrients-rich "biofortification”wheat[18]. Several synthetic hexaploids have higher concentrations of both micronutrients and macronutrients[19]. Zinc-rich and high-yielding SDLs have been developed at CIMMYT[20], and a number of them have been registered as cultivars in India under the names Zinc Sharkti, WB2, and HPBW 01 (Table 1). More SDL-derived cultivars will be released for this purpose.
China is one of the major countries that takes full advantage of SHW as a genetic resource, especially in Sichuan Province of south-west China. Since the introduction of over 200 SHW accessions from CIMMYT in 1995, four SDL-derived cultivars, Chuanmai 38, 42, 43, and 47, have been generated. These have been registered and released to farmers for large-scale growth [21]. Among them, Chuanmai 42 was recorded as a cultivar with the highest average yield (>6 t · hm-2) in Sichuan Province for two consecutive years. It has large kernels and is resistant to stripe rust, which is caused by Puccinia striiformis ( P.striiformis). These Sichuan SDLs have been widely used in wheat breeding. For example, from Chuanmai 42 and Chuanmai 43, a number of secondary synthetic derivative cultivars have been developed and released, including Chuanmai 51, 53, 56, 58, 61, 64, 104, Mianmai 51, 228, 367, 1618, and Shumai 969 [21,22] (Table 1). Shumai 969 contains the additional synthetic wheat SHW-L1 that is derived from the Chinese tetraploid wheat accession AS2255, a T.turgidum ssp. turgidum accession, and the Iranian Ae.tauschii ssp. tauschii accession AS60. The whole pedigree of this variety is SHW-L1/ Chuanmai 32//Chuanyu 16/3/Chuanmai 42. Another SHW-L1-derived SDL, Shumai 580, which was bred in Sichuan Province, has now been preregistered in Yunnan Province, demonstrating the wide adaptability of these cultivars. Despite the success of SHWs in commercial wheat development, the total number of SHWs that have been utilized is still fairly low, especially in other major wheat-growing regions in China such as Shandong and Henan Provinces; thus,additional effort should be put into exploring the utilization of SHWs.
《Table 1》
Table 1 List of synthetic wheat and derived cultivars that have been released for breeding.

Table 1 (continued)
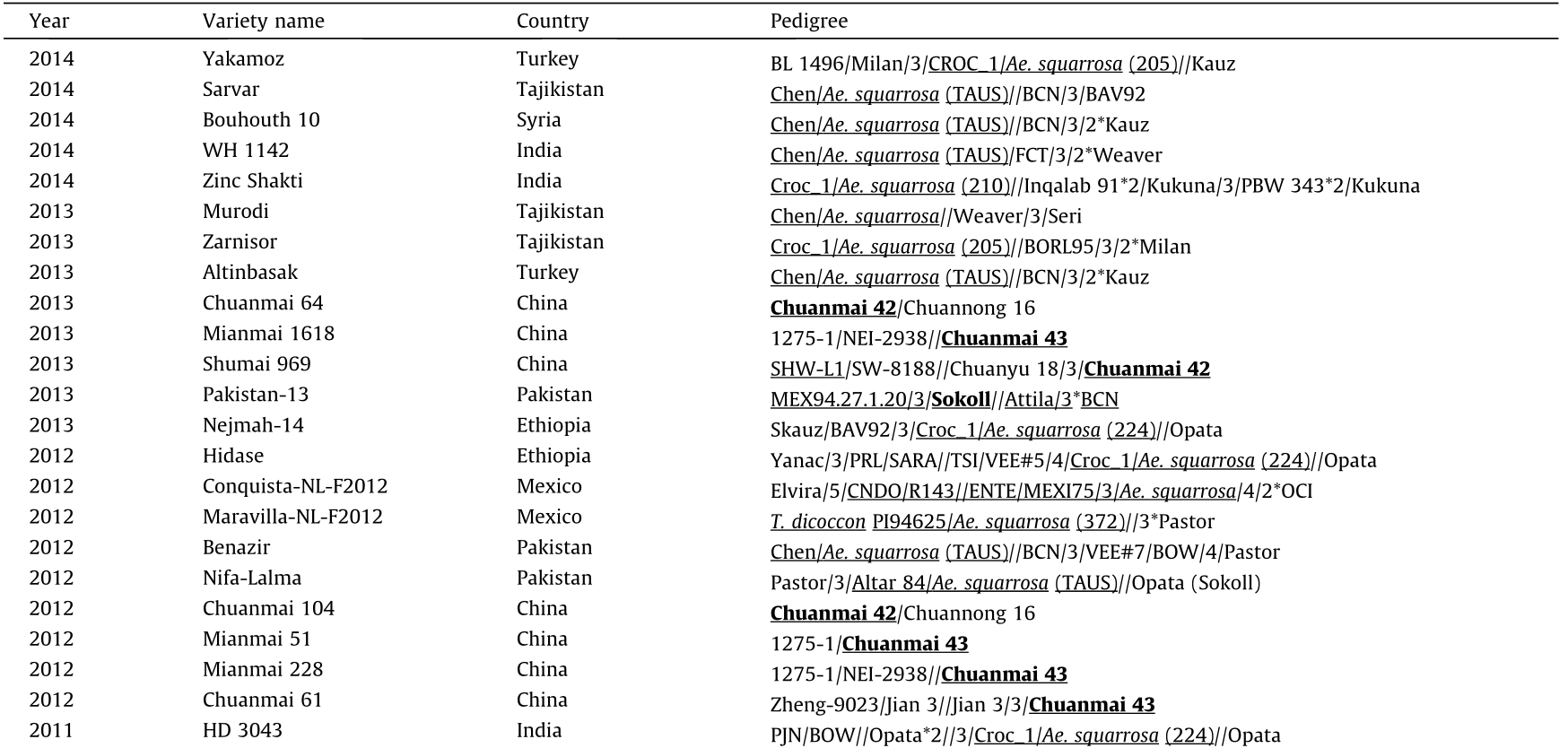

In pedigrees, synthetic wheat or their parental combinations are underlined; synthetic wheat derivatives are bolded and underlined. /: cross with; // :second cross; /3/ :third cross; /4/: forth cross; /5/: fifth cross; *:times.
a The primary synthetics were derived from T.dicocccum instead of T.durum.
《3.SHWs are valuable resources for wheat genetic improvement》
3.SHWs are valuable resources for wheat genetic improvement
《3.1.Resistance to biotic stresses》
3.1.Resistance to biotic stresses
The availability of SHW provides an opportunity to seek new resistance to many pathogens. Novel resistance genes for leaf, stem, and stripe rusts have been reported in synthetic wheat, including Lr32 for leaf rust (caused by P.recondite Erikss.) [23], Sr33 and Sr45 for stem rust (caused by P.graminis f.sp.tritici) [24,25], and Yr28 for stripe rust [26]. Many SHWs have also demonstrated resistance to other severe fungal diseases, including Stb5 and Stb17 for Septoria tritici blotch [27,28], Tsr3 for yellow leaf spot (caused by Pyrenophora tritici-repentis) [29,30], and Pm2 and Pm18 for powdery mildew (caused by Blumeria graminis f.sp.tritici)[31]. New resistance genes were also found for insect pests such as GB3 and GB7 for green bug [32,33], DN3 for Russian wheat aphid(Diuraphis noxia Kurdjumov) [34], Cmc1 for wheat curl mite (Aceria tosichella Keifer)[35], and H13, H26 for Hessian fly (Mayetiola destructor(Say)) [36,37].
The goat grass Ae.tauschii is a valuable source for multiple disease-resistance genes, with many loci for disease resistance being reported in an association analysis of CIMMYT SHW [38,39]. Among 37 synthetic hexaploids with high grain weight that were selected from the CIMMYT Elite-1 subset collection, Kazi et al. [40] showed that all had resistance to Karnal bunt (caused by Tilletia indica Mitra)and 62%of them possessed resistance to prevailing races of P.striiformis,the causal agent of stripe rust in wheat; this makes them available to improve the yield potential of common wheat in areas where these two biotic stresses are the main constraints for wheat production. In addition, the stripe rust resistance gene YrAS2388 is a common source of stripe rust resistance in Ae. tauschii accessions from the Caspian Sea region [41]. It has been mapped on chromosome arm 4DS in Ae.tauschii [42,43], which may be the same as the gene Yr28 mapped from a SHW line [44]. Despite this, many Ae.tauschii accessions used for making SHW are stripe rust susceptible. This means that some of the resistance genes in SHW may come from durum wheat. This is likely because resistance genes for stripe rust and Karnal bunt have been reported in the durum parents of SHW, including the key stripe rust-resistance gene YrCh42 of Chuanmai 42 [45]. It must be noted,however, that there exist suppressor genes of resistance in both tetraploid wheat and Ae. tauschii, and some traits may be suppressed in synthetic wheat after hybridization [46].
《3.2.Resistance to abiotic stresses》
3.2.Resistance to abiotic stresses
Ae.tauschii and SHW are potential sources of new genetic variation for abiotic stress tolerance in wheat improvement.Studies showed that SDLs may provide up to 45% yield increase over their common wheat parents under drought conditions [47]. Increases of 8% –30% in yield, in comparison with parental lines and local check varieties,were achieved under rainfed conditions in Australia [48]. Similar yield increase was also achieved in India, Pakistan ,Ecuador, and Argentina. These synthetic varieties bear thicker roots that can grow deeper into the soil,which is important in dry environments.Additional genetic variation for salt tolerance has been reported in SHW [49,50], which is limited in elite common wheat genotypes. Moreover, it has been shown that at the grain-filling stage, synthetic wheat can tolerate higher temperatures of up to 35–40°C [51]. Using 33 CIMMYT SHW lines and their SDLs, Jafarzadeh et al. [52] showed that SHW may contribute to yield improvement in SDLs under heat stress conditions and may perform even better than under drought or irrigated conditions.
Again, different sets of D genome genes may underlie the differences in the abscisic acid (ABA) responsiveness of SHW. In light of the role of ABA in plant adaptation to environmental stresses, Ae.tauschii accessions with high ABA-sensitivity should be adopted in common wheat abiotic stress-tolerance improvement [53].
《3.3.Pre-harvest sprouting》
3.3.Pre-harvest sprouting
Pre-harvest sprouting (PHS) is a particularly important issue in East Asia, where high rainfall and humidity often occur before and/or during the harvest-season, resulting in downgraded wheat flour and financial losses to farmers. Ae. tauschii has greater seed dormancy with a major component for PHS resistance (PHSR) [54,55]. Synthetic backcross lines (SBLs) were derived from a PHSR SHW line that was backcrossed several times with a non-PHSR common wheat line. These genetic materials are important in producing elite wheat varieties by introgression and PHSR gene pyramiding. Such a strategy allows the development of white PHS-resistant wheat germplasms from Ae.tauschii accessions with red grains that are usually considered to be more resistant to PHS [56].
《3.4.Yield and yield components》
3.4.Yield and yield components
Ae.tauschii has a number of yield traits or components that may be transferred to SHW when used as a paternal parent. Ae. tauschii accessions display wide variation in grain size and shape. These traits are among the main targets of modern wheat breeding. In order to characterize the quantitative trait loci (QTL) underlying these traits, Okamoto et al. [57] developed four F2 mapping populations using SHW lines. The researchers then measured six parameters related to grain size and shape among the progeny. A total of 18 QTL were identified to underlie these traits, which distributed on five out of the seven chromosomes. This work indicates that at least some QTL from Ae. tauschii are functioning in SHW, and are therefore usable for common wheat yield improvement.
Although SHW seems to have a lower yield potential relative to common wheat cultivars, it usually shows higher above-ground biomass that may convert to higher grain yield. This biomass may also increase the harvest index and grain weight [58–60]. Larger grains along with a greater number of seeds per spike and tillers were transmitted to SDLs [61]. Moreover, synthetics with high combining ability for yield also had high combining ability for spike number or seed number per spike. Improved yield could result from selecting lines with increased seed number per spike among those derived from synthetic wheat populations. It has been shown that SDLs confer up to 11% higher yield than the common wheat parental lines [62]. In a SHW winter wheat cultivar (Karl 92) cross, BC2-derived populations had up to 30% higher grain yield than Karl 92 [63]. Chuanmai 42 , an SDL variety, even gave 22.7% higher yield than the commercial check, Chuanmai 107 [64]. Analysis with genetic markers showed that chromosome 4D of the SHW parent was responsible for the yield increase in Chuanmai 42 [65,66]. In CIMMYT, the first SDLs appeared in the international nurseries (i.e.,sets of the best wheat lines for international distribution) in the second half of the 1990s. Even though the numbers have varied in different nurseries and years, the percentage of SDLs in the semiarid wheat yield trial (SAWYT) hit 52% in 2010,with a five-year average (2011–2015) of 35%. These data indicate that SDLs are competitive resources worldwide and carry both broad adaptive characteristics and unique yield improvement components [67].
《4.Understanding the molecular mechanism underlying the growth vigor in SHW》
4.Understanding the molecular mechanism underlying the growth vigor in SHW
Hexaploid wheat confers better grain quality and adaptability than its progenitors. Newly synthesized allohexaploid wheats are good models for studying the molecular basis that underlies wheat improvment traits [68]. The grain length of newly made SHW is often similar to that of T.turgidum, and so are traits such as plant height and architecture, flour characteristics such as endosperm starch composition,and biotic stress features such as resistance to powdery mildew.Like its tetraploid parent, SHW often exhibits a spring flowering habit [68]. In addition, more robust growth is observed in seedling size, seed size,and spike size. With its genetic stability and fixed heterozygosity, SHW is an ideal material to study the molecular mechanisms of growth vigor in polyploid plants.
《4.1.Genome alterations in SHW》
4.1.Genome alterations in SHW
Both allotetraploid and allohexaploid wheats can be reproduced in the laboratory, allowing tractable study of the genetic, functional,and epigenetic changes in SHW genomes caused by polyploidization. Allohexaploid wheat can be synthesized by interspecific hybridization followed by spontaneous chromosome doubling [69]. For successful synthesis of allotetraploid wheat, colchicine treatment is compulsory for chromosome doubling [70]. Early studies documented that the onset of both allotetraand allohexa-polyploidization events in wheat was associated with rapid and extensive changes in genomes such as DNA rearrangements [70]. Recent work suggests that most structural changes may have taken place during allotetraploidization [69,71,72]. Molecular study showed that,compared with their parental lines, changes in genome structures and copy numbers of certain genes occurred in SHW lines [73]. Despite this,SHW lines with euploid chromosomes are genetically stable and are suitable for studying the molecular mechanisms underlying their growth vigor [69,72,74].
《4.2.Gene expression changes in SHW》
4.2.Gene expression changes in SHW
New technologies such as microarray and RNA-Seq have been applied in the comparative study of SHW, modern variety,and their direct parents [75–81]. A naive comparison was first conducted,in which gene expression levels in SHW were compared with those of mid-parent values (MPVs) that are derived by averaging the expression levels of genes from the two parental lines [82]. However, additivity, rather than non-additivity, was found to be pervasive [77,78]. Such an observation requires alternative methodology to measure gene expression differences between different species.In this regard, Li et al. [68] applied the concept of" expression-level dominance”to study gene expression patterns during allopolyploidization [83]. Based on this concept, a functional dominance of genes in the SHW with similar expression levels to those in T.turgidum were enriched for plant development.By the same concept, genes with similar expression levels to those in Ae. tauschii were found to be involved in adaptation. Such a pattern suggests that subfunctionalization of genes from the two parental lines has occurred to some extent in SHW [68].
《4.3.Dynamics of small RNA expression levels in SHW》
4.3.Dynamics of small RNA expression levels in SHW
Small RNAs are 21–24 nucleotide non-coding RNAs that regulate gene expression by post-transcriptional mechanisms and through epigenetic modifications [84,85]. They are classified into micro RNAs (miRNAs) and small interfering RNAs (siRNAs). miRNAs regulate target gene expression by directly cleaving its transcripts or by translation repression. Studies in model plants such as Ara-bidopsis provide good lessons for crops such as wheat. It has been shown that in Arabidopsis, non-additive expression of miRNA target genes may be caused by variation in miRNA expression levels. Changes in miRNA expression level may be responsible for Arabidopsis growth vigor and adaptation [86]. On the other hand, siRNAs may mediate DNA methylation, which dynamically regulates the expression of genes with a transposable element (TE) in their neighborhood, and hence may serve as an absorber against the shock caused by the merging of different genomes in one plant. siRNAs may have a parental effect and preferentially repress homeologs from one parent [82,87,88], causing biased expression of genes in the allotetraploid progeny. Common wheat and its progenitors,as well as SHW, are rich in repetitive sequences (>80%) that are mainly derived from various TEs. The fact that the number of siRNAs corresponding to TEs strongly decreased upon poly-ploidization may suggest a significant role of TEs in this process [89]. Li et al. [68] also discovered that a high proportion of miRNAs was non-additively expressed upon polyploidization. It is possible that changes in miRNA expression level may be caused by the non-additive expression of target genes that are important for growth vigor in SHW. Li et al.[68] also found that the density of siRNAs from the D subgenome was increased in SHW. It is likely that these increased siRNAs may repress D homeolog expression,thus causing biased expression of AB homeologs in SHW. These molecular underpinnings, together with those described above that are established during early allopolyploidization events, may lay the groundwork for the successful advent of common wheat.
《5.Perspectives》
5.Perspectives
Allopolyploidization involves both hybridization and poly-ploidization. Genome interactions may cause complex changes in allopolyploid plants at the genetic, epigenetic, biochemical, and regulatory network levels. Studies of changes in genetic frame works may reveal biochemical mechanisms working in this process. An integrative approach combing data from genome wide association studies (GWASs), transcriptomics, epigenomics, proteomics, and metabolomics should be adopted. The results can shed new light on the molecular mechanisms underlying the vigor phenotypes of SHW at different developmental stages that is, larger spikes, larger grains, and greater biomass. Along with such a systems approach, regulatory networks should be dissected individually so that their contribution to individual vigor traits can be assessed. Key genes in the regulatory pathway can be experimentally tested using additional methods such as transgenic tools [90].
Synthetic wheat is a useful genetic resource that should be utilized to transfer needed agronomically important genes from a wider range of tetraploid or diploid donors, including wild species, in order to improve the performance of common wheat. Genomics and functional genomics studies can assist us in better understanding the molecular bases for SHW growth vigor. A number of refer-ence genomes, for both common wheat and its progenitors, have recently been made available and should facilitate the study of SHW from a genomic point of view. The draft genome sequences of the A and D donor genomes were made available several years ago [91,92] when multiple versions of hexaploid wheat genomes were under development [93]. In 2017, the genome of the wild emmer wheat (T.turgidum ssp.dicoccoides) became available, providing details on the gene content, genome architecture,and genetic diversity of the tetraploid donor of common wheat [94]. Moreover, the reference grade hexaploid wheat genome is soon to be available [95], together with further improved diploid ances-tor genomes [96]. The release of these genomics information should further accelerate functional genomics research in wheat.
In light of the global demand for continuous wheat production to keep up with the world’s increasing population, a significant yield jump like the Green Revolution is desirable.Wheat yield, however, is reaching a plateau. To make things worse,global warming is becoming a worldwide threat,with more severe drought and heat occurring at higher frequencies. In addition, new pathogen strains(particularly rusts)and diseases are emerging more frequently,posing further problems for wheat production. In 2010, a stripe rust race(a breaking down of the Yr27 resistance gene) damaged about one third of Ethiopian wheat fields. In 2013, Ethiopia was struck again by stem rust that caused similar significant loss in wheat production [97]. Wheat blast, caused by the rice blast fungus Magnaporthe oryzae, has now appeared in Bangladesh, where about 16% of the cultivated wheat area was wiped out in 2016 [98]. These dispairing circumstances call for a new type of‘‘super wheat”with a high yield and robust stress tolerance. Synthetic wheat, equipped with its broad genetic resources from wild donor species, is poised to play a bigger role in the race to meet upcoming environmental challenges.
《Acknowledgements》
Acknowledgements
The work related to synthetic wheat is supported by National Natural Science Foundation of China (31661143007 and 31571665) and the Major Breeding Program from Ministry of Science and Technology of China (2016YFD0101004 and 2016YFD0102002).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Aili Li, Dengcai Liu ,Wuyun Yang, Masahiro Kishii,and Long Mao declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




