《1. Introduction》
1. Introduction
Lesions and defects that require tissue or organ transplantation remain urgent problems in clinical medicine [1], and problems still exist regarding the use of current approaches, which include autotransplantation, xeno-transplantation, and the implantation of artificial mechanical organs. Although auto-transplantation can yield a satisfactory effect, the positive result is at the cost of antilogous health organization, and may lead to many complications and secondary injuries. Potential risks are also associated with xeno-transplantation, such as immunological rejection and viral transmission; furthermore, source donors are limited. Nevertheless, the implantation of artificial organs in clinical treatments is often successful and improves the quality of life of patients. Three-dimensional (3D) printing technology is expected to solve the limitations that are inevitably encountered when using traditional methods by incorporating the personalized construction of human bionic tissue or organs.
3D printing, also known as additive manufacturing, is based on the principle of layered manufacturing, in which materials are overlapped layer by layer. This technology can be used to quickly fabricate components with any complex shape by accurately accumulating material using solid modeling according to a computeraided design (CAD) model or computed tomography (CT) scan under computer control [2]. The 3D printing industry has recently exploded due to the reduced manufacturing costs of 3D printers and to their improved printing precision and speed, allowing for huge advances in medical equipment, implant material, and cell printing. The preparation of organ models, rapid manufacturing of personalized scaffolds, and direct printing at the defect site can be achieved by 3D printing technology based on a patient’s imaging data such as CT or magnetic resonance imaging. In this way, 3D printing technology brings new possibilities for building bionic tissue or organs and solving the donor-shortage problem.
Current research on 3D printing technology for medical applications can be classified into the following four main areas of focus: ① research on manufacturing pathological organ models to aid preoperative planning and surgical treatment analysis [3]; ② research on personalized manufacturing of permanent nonbioactive implants; ③ research on fabricating local bioactive and biodegradable scaffolds; and ④ research on directly printing tissues and organs with complete life functions [4–6]. Although such applications remain far from widespread clinical use due to several key technical and basic scientific issues that are yet to be overcome, notable scientific advancements and applications have been achieved in these areas.
《2. Four levels of 3D printing for medical applications》
2. Four levels of 3D printing for medical applications
《2.1. Organ models to aid preoperative planning and surgical treatment analysis》
2.1. Organ models to aid preoperative planning and surgical treatment analysis
High-fidelity physical organ models play a significant role in clinical treatment and in medical education. Conventional manufacturing processes, such as casting or forging, waste so much time in preparing expensive tooling, and always ignore individual differences among patients [7]. 3D printing has the advantage of rapidly fabricating customized medical models at a lower cost, since there are no tools involved. 3D printed organ models primarily help doctors to perform surgical analysis and preoperative training. Personalized medical models with complex shapes that are made using 3D printing can provide doctors and engineers with a medium for communication, and can assist in surgical planning and diagnosis. There is no requirement for biocompatibility of materials in such applications, which include medical models and in vitro equipment for preoperative planning, prosthesis design, testing standards, and so on, because printed parts will not enter the body.
A group of researchers from China and America have used 3D printing technology with HeLa cells and gelatin/alginate/fibrinogen hydrogels to successfully construct in vitro cervical tumor models [8], thus providing vivid 3D imaging of the tumor environment. As shown in Fig. 1 [8], HeLa cells form round spheroids with smooth surfaces and tight cell–cell connections within the 3D hydrogel, while exhibiting a flat and elongated morphology on 2D tissue culture plates. Compared with 2D planar cultures, models in 3D constructs can reveal the characteristics of tumor cells so that researchers and clinicians can better recognize the proliferation, differentiation, and spread of the tumor.
《Fig.1》

Fig. 1. Cellular morphological changes in a 3D construct and 2D planar culture. (a) HeLa cells for different periods; (b) distribution of the cytoskeleton after staining; (c) distribution of spheroid diameter in 3D HeLa/hydrogel constructs [8].
Researchers at Monash University, Australia, developed a unique 3D printed human anatomical model made from gypsumlike powder or plastic by scanning real anatomical specimens using CT or a planar laser scanner. This series of high-resolution 3D printed anatomical replicas, including limbs, chest, abdomen, head and neck, and other major body parts (Fig. 2), is available for anthroponomy training in medical colleges or hospitals.
《Fig.2》

Fig. 2. 3D printed human anatomical model kits, including (a) head and (b) arm, published by Monash University.
Not only can 3D printed models show the actual condition of various tissues and organs in vivo, but personalized medical models can also help doctors investigate patients’ states and prepare preoperative plans. Cardiologists from Spectrum Health Helen DeVos Children’s Hospital in Grand Rapids, Michigan announced that they have 3D printed a more detailed patient cardiac module (Fig. 3) using synthetic data from multiple imaging technologies, which sets the precedent for utilizing both CT scans and 3D echocardiography data to print a 3D mixed model. Previous 3D printing methods simply used an imaging modality, whereas hybrid 3D printing can assist in correct diagnosis and surgery planning, as it can be used to simulate the surgical procedure and reduce complications by integrating various imaging methods and corresponding data sets.
《Fig.3》

Fig. 3. (a) CT scan and 3D echocardiography data model; (b) 3D printed cardiac module combining various medical imaging technologies.
3D printed models are more competitive than plastinated specimens in terms of physical dimension and durability; they can even be color- or material-coded by tissue type. However, in addition to the higher price and 3D data acquisition of such models, problems such as limited printing resolution, longer printing time, and a few available multi-materials similar to the intended organ or tissue during the process need to be solved [9]. To improve the effectiveness of 3D printed models, researchers can further develop materials with different tactile elasticities, colors, and composition in order to simulate the appearance of human tissues and organs, and thus allow people to gain tactile experience from models [10].
《2.2. Permanent non-bioactive implants》
2.2. Permanent non-bioactive implants
Permanent medical implants commonly used in dentistry and orthopedics require non-degradable biomaterial and offer good biocompatibility after surgical operation. Compared with fabricating implants by means of traditional machining technology, 3D printing can achieve personalized real-time manufacturing of any complex implant with high dimensional accuracy and short production cycles. During traditional bone treatments, stressshielding phenomena can easily occur because traditional metallic implants present greater stiffness than bone, which will eventually compromise bone integrity. Integrating topology-optimization designs with 3D printing is a new and effective technology to fabricate lightweight customized implants with adjusted stiffness [11]. This technology is also highly compatible with digital measuring devices that have been widely used, in terms of data conversion and space docking.
Zhang et al. [12] designed and manufactured custom joint prostheses according to the osseous morphology of resected proximal tibial osteosarcomas. Fig. 4 [13] shows postoperative imaging of a custom prosthetic reconstruction. In that study, most patients recovered excellent motor function with few complications; however, more attention should be paid to the long-term functional recovery of patients. Thus, custom joint prosthetic reconstruction has demonstrated satisfactory results for patients with osteosarcoma in the proximal tibia or the proximal tibiofibular joint during neoadjuvant chemotherapy and limb salvage operations. Galasso et al. [13] also implanted a custom distal femur modular prosthesis into a 20-year-old patient with stage IIB osteosarcoma. Ten months after surgery, the knee range of motion reached 0–110°, and the patient could bear full weight painlessly and without support.
《Fig.4》

Fig. 4. Postoperative (a) anteroposterior and (b) lateral radiographs of custom prosthetic reconstruction [13].
Current technology for the 3D printing of permanent implants is relatively mature; thus, the present study focuses on three aspects: First, the development of medical materials with better performance is discussed. For example, Winder et al. [14] fabricated custom cranial titanium (Ti) implants made directly from stereolithographic resin (Fig. 5(a)), thus greatly simplifying the process. Second, we present the uses of advanced technology to manufacture implants with excellent mechanical properties matching those of bone. For example, SLM Solutions in Germany fabricated a Ti hip implant (Fig. 5(b)) for an Australian patient using a selective laser melting (SLM) process. This high-strength and lightweight implant had good compatibility with human tissue. Third, improving the biocompatibility and preventing infections of existing mature medical materials by surface modifications in order to meet medical requirements are overviewed. As shown in Fig. 6(a), Bian et al. [15] from Xi’an Jiaotong University associated hydroxyapatite (HA)-coated porous Ti, which is regarded as the carrier, with bone morphogenetic protein-2 (BMP-2) via gelatin. They successfully prepared 3D porous Ti with osteoconductivity composed of an osteoinductive composite material. Compared with the tissue slices in the control group (Fig. 6(b)), Fig. 6(c) and Fig. 6(d) show that more bone mass newly formed in the BMP-2 gelatin/HA porous Ti group after 6 weeks, and that the surfaces of new bone are in direct contact. After 24 weeks, the porous Ti was full of new bone, effectively promoting early healing of bone. In addition to HA, other hydrogel components can be used to coat alloy scaffolds. Sing et al. [16] integrated SLM with collagen infiltration to build biphasic scaffolds with Ti/type 1 collagen and Ti-Ta/type 1 collagen. The collagen component plays a role in cell differentiation and growth, promoting bone regeneration and vasculature, while the scaffold component provides the required mechanical strength.
《Fig.5》

Fig. 5. (a) Stereolithographic resin model of a full skull with a custom Ti plate in place; (b) hip implant fabricated by SLM Solutions in Germany [14].
《Fig.6》

Fig. 6. (a) X-ray film of BMP-2 gelatin/HA porous Ti complex; (b) tissue slices observed at 6 weeks after implanting HA-coated porous Ti; (c) tissue slices observed at six weeks after implanting BMP-2 gelatin/HA porous Ti complex; and (d) tissue slices observed at 24 weeks after implanting BMP-2 gelatin/HA porous Ti complex [15].
Surface modification can even inhibit bacterial adhesion and long-term biofilm formation, which may cause infection. Researchers have found that although host defenses and conventional antibiotics can eliminate infections caused by planktonic bacteria, they are powerless against bacteria-forming biofilms. Avoiding initial bacterial adhesion to form biofilms is crucial in reducing the infection risk of implants [17]. Recent research shows that tailoring the antibacterial properties of the implant surface—mainly by chemical modification of biomaterials via zwitterionization—and special surface nanotopography or architecture are effective for controlling and preventing bacterial adhesion of implants. Zong et al. [18] designed and synthesized zwitterionic HA with bacterial antiadhesive properties and in vitro biocompatible behavior via direct-write assembly. The efficient interaction of these zwitterionic pairs on the HA surface reduces bacterial adhesion by more than 90%, compared with unmodified HA, and the in vitro biocompatibility of HA is preserved in the experiments. Izquierdo-Barba et al. [19] also found that a Ti6Al4V alloy coated with Ti nanostructures by means of the glancing angle deposition technique using magnetron sputtering (MS-GLAD) has a high nanocolumnar density, and exhibits strongly impaired bacterial adhesion that inhibits biofilm formation. These methods of preventing bacterial adhesion offer new directions for 3D printed permanent implants to meet medical requirements.
《2.3. Fabricating local bioactive and biodegradable scaffolds 》
2.3. Fabricating local bioactive and biodegradable scaffolds
There are two possible routes for manufacturing tissues and organs, depending on whether cells are directly manipulated during the formation process. The first route is tissue engineering (Fig. 7(a)), also known as indirect cell assembly, which involves first forming a 3D scaffold, and then seeding cells [20]. Alone or combined with living cells, biocompatible materials, growth factors, and physical factors can be used to create a biomimetic tissue-like microarchitecture scaffold [21]. The second route, known as direct cell assembly, formulates both cells and materials into a composite structure [1]. As shown in Fig. 7(b) and Fig. 7(c), the mixture of cells and gel is encapsulated into 3D scaffolds that are composed of another kind of gel with good mechanical strength, or are printed directly in order to control the spatial distribution of cells and even realize in situ repair. Biodegradable scaffolds play an important role as a bionic structure of the extracellular matrix. Compared with traditional scaffold-fabrication methods, 3D printing can manufacture any complex structure with both microscopic pores and macroscopic shapes, and allows effective control of the microstructure and physicochemical properties of scaffolds.
《Fig.7》

Fig. 7. Schematic views of three processing methods for personalized biodegradable scaffolds. (a) encapsulating cells directly into gel scaffolds; (b) encapsulating cells/gel mixture into 3D scaffolds; and (c) directly printing cells/gel.
As carriers of cell attachment, scaffold materials must exhibit good biocompatibility and biodegradability, and the scaffolds must react with human tissue to promote tissue regeneration after implantation. Hydrogels can further aid cell migration and growth to improve the speed of tissue regeneration and repair, by acting as a new type of functional material with bionic characteristics that resemble those of the extracellular matrix with highly 3D network structures. A growing number of researchers have realized the use of these biomaterials as carriers for transplanting cells and for the delayed release of growth factors, since the characteristics of hydrogels make them particularly appealing for repairing and regenerating soft tissues and organs [22]. When repairing tissues, researchers pay a great deal of attention to enhancing or accelerating vascularization, which is a major limitation. Thus, one research group [23] has designed a new concept in which a hybrid scaffold is composed of thin porous membranes and filament meshes that alternate in layers in order to prompt the growth of blood vessels. To improve the layer-by-layer pattern of 3D scaffolds and broaden the network design space, 3D biomimetic microvascular networks can even be printed by direct-write assembly within a photocurable gel reservoir in order to produce the desired microvascular network [24]. In situ printing of 3D scaffolds is feasible and effective for promoting bulk tissue regeneration. For example, fibrin/collagen hydrogel can be printed above the fibroblast layer by simultaneous deposition of thrombin from atomizing nozzles [25]. The final complete re-epithelialization of the large wound in this study shows that in situ skin printing could be used for the immediate repair of wounds.
2.3.1. Characteristics of scaffolds
3D printed personalized scaffolds for clinical applications can be applied to treat various symptoms that differ among patients. These scaffolds require bio-physicochemical properties, structural features, mechanical properties, and so on with the following characteristics [26]: a 3D porous interconnected network for cell growth and flow transport of nutrients and metabolic waste; biocompatibility and matching with the controlled degradation and absorption rate of cell/tissue growth in vitro and/or in vivo; suitable surface chemistry for cell adhesion, proliferation, and differentiation; and properties that match the tissues to be implanted.
2.3.2. Classification of scaffolds
(1) Indirect cell assembly: encapsulating cells directly into gel scaffolds. For indirect cell assembly, the cells are seeded into porous gel scaffolds (Fig. 7(a)). Primary classification of rapidly manufactured scaffolds with high water content, excellent biocompatibility, and controllable biodegradation can be made based on the principal technique that was used to form the scaffold [27]: ① extrusion-based, ② inkjet-based, ③ microvalvebased, and ④ laser-assisted bioprinting. The extrusion-based bioprinting technique combines a fluid-dispensing system and an automated robotic system for extrusion and bioprinting. Ozbolat and Hospodiuk [28] used a deposition system to realize the precise deposition of cylindrical filaments with seeded cells to form desired 3D custom-shaped structures controlled by computer. Inkjet-based bioprinting (including continuous inkjet bioprinting and drop-on-demand inkjet bioprinting) has great advantages for simplicity and agility, with precise control of the deposition of cells and biomaterials. It leverages gravity, atmospheric pressure, and fluid mechanics to physically manipulate bioink to generate droplets, and then eject them onto a receiving substrate [29]. Microvalve-based bioprinting is also a kind of drop-on-demand printing, but is a more reliable system for high-throughput printing compared with inkjet-based bioprinting [30]; it can realize the precise deposition control of multiple cells and biomaterials with high cellular viabilities and high throughput rates, and has a moderate printing resolution. Unlike the first three bioprinting methods, the substrate of laser-assisted bioprinting is usually coated with a thin layer of laser-absorbing material and a second thicker layer of biomaterial, such as hydrogel with embedded cells. Laser pulses are focused into the laser-absorbing layer; the evaporation of this layer in the laser-focused region then generates a high-pressure vapor bubble. This bubble moves biomaterials forward by vapor pressure, and the biomaterials are then deposited as a droplet at a predefined position on the collector slide [31].
Due to the advantages of the rapid 3D printing of fine structures by combining CT images, CAD/computer-aided engineering (CAE) functionalized biomimetic structural design, and gel/cell hybrid processes, personalized cell-loaded scaffolds prepared by different techniques have achieved good results in cell experiments and surgical implants. Table 1 [32–41] summarizes international research and recent advances in cell-loaded hydrogen scaffolds.
《Table 1》
Table 1 International research and recent advances in personalized scaffolds prepared by different techniques

GelMA: gelatin methacrylate; PSL: projection stereolithography; HUVECs: human umbilical vein endothelial cells; 3DP: three-dimensional printing; nHA/PLGA: nanohydroxyapatite/poly lactic-co-glycolic acid; PCL: polycaprolactone; FDM: fused deposition modeling; PPF: poly propylene fumarate; SLS: selective laser sintering; PU: polyurethane; HCs: hepatocytes; dECM: decellularized extracellular matrix.
(2) Direct cell assembly: encapsulating cells/gel mixture into 3D scaffolds. Directly seeding cells into 3D printed scaffolds will cause a low inoculation rate and an exponentially expanding celldistribution space [42,43]. To solve these problems, researchers encapsulate a cells/gel mixture into scaffolds that are composed another kind of gel in order to obtain the advantages of the good mechanical strength and shapeability of 3D scaffolds (Fig. 8). Hydrogels also play a role in homogeneous encapsulation and cell adhesion based on this method. Hematoxylin-eosin staining slice (Fig. 8(b)) and safranin O staining slice (Fig. 8(c)) show abundant evenly distributed cells with lacuna, forming island cartilage tissue.
《Fig.8》

Fig. 8. PLGA-collagen gel with chondrocytes implanted for 12 weeks under a light microscope. (a) Hematoxylin-eosin staining (× 40); (b) hematoxylin-eosin staining (× 100); (c) safranin O staining (× 40).
Uniformly and efficiently embedding chondrocytes into scaffolds provides a suitable 3D growth environment for chondrocytes by simultaneously combining the strength of the collagen gel with certain mechanical properties of the rapid-prototyping poly lactic-co-glycolic acid (PLGA) scaffolds. As shown in Fig. 9, spindle cells existed on the surface of the specimen, and multilayered round cells have formed cartilage lacunae in the deep layer. Most of the close-packed cells were matured, and the majority of the equally distributed cells were rounded with lacunae. Cartilage matrix formed in the pores of the biomaterials, promoting island cartilage tissue. Visible pores remained after the scaffolds had completely degraded. Hong et al. [44] encapsulated human embryonic kidney (HEK) cells into a type 1 rat-tail collagen solution, and then gelled the mixture to the interconnected pores of a printed poly(ethylene glycol) (PEG)–sodium alginate–nanoclay network to form a synthetic hydrogel. Seeded cells maintained high viability after seven days of culture (Fig. 9(c)), confirming that tough hydrogels containing nanoclay are biocompatible and suitable for long-term cell culture.
《Fig.9》

Fig. 9. (a) A network printed with tough, biocompatible hydrogel; (b) live-dead assay of human embryonic kidney (HEK) cells in a collagen hydrogel seeded in the 3D printed network; (c) viability of HEK cells after seven days [44].
(3) Direct cell assembly: directly printing cells/gel. Encapsulating cells directly, or a cells/gel mixture, into 3D scaffolds can only simulate a bionic structure of the extracellular matrix in vitro. Implanting a scaffold into the injury could even cause secondary damage and complications. In order to realize in situ repair in vivo, many researchers have encapsulated cells directly into hydrogels to simultaneously control cell growth and spatial distribution (Fig. 7(c)). Hockaday et al. [45] used a mixture of poly(ethylene glycol)-diacrylate (PEG-DA) and alginate combined with porcine aortic valve interstitial cells (PAVICs) for printing. Fig. 10 [45] shows that the cell viability of PAVICs in the scaffolds was maintained near 100% over 21 days. This method can be used to rapidly fabricate anatomical heterogeneous valve conduits for cell implantation. Xue et al. [46] mixed human dental pulp cells and alginate/gelatin gel to manufacture scaffolds with customized shapes and sizes according to certain parameters, using a bioprinter. Cell density was controllable, and viable cells with high viability adhered within the 3D network. This research established the foundation for bioprinting technology with further applications in tooth tissue engineering, and could provide a new method for tooth regeneration.
《Fig.10》

Fig. 10. Live-dead imaging of PAVICs. (a) Live PAVICs were visible in both the root (top) and leaflet (bottom) for different periods; (b) cells detected in the interstitium of the root and leaflet over 21 days [45].
In addition to using 3D printing technology to fabricate living biological constructs in vitro, hydrogels can be printed directly at the wound site for in situ repair; this means that parts with complex damage can be repaired rather than replaced, and new approaches toward minimally invasive repair can be explored. For example, alginate hydrogel and a novel formulation of demineralized bone matrix have been used for the in situ repair of cartilage and bone, respectively; the surface errors of the repair points were within a clinically acceptable error range (Fig. 11) [47]. In addition to orthopedic repair, this technique can be extended to other biomedical fields, such as minimally invasive and personalized facial reconstruction, by choosing other materials and appropriate cells for seeding. Cui et al. [48] also made significant progress in cell/gel in situ prints. They prepared a mixture of poly(ethylene glycol) dimethacrylate (PEGDMA) and human articular chondrocytes as bioink, and then constructed in situ prints in cartilage defects using ultraviolet (UV) light. Cells were equally distributed in the gel scaffolds after printing and maintained a high viability. Table 2 indicates that the compressive modulus of the printed hydrogel was near the range of properties of native human articular cartilage, and thus represents a promising material for anatomic cartilage engineering.
《Fig.11》

Fig. 11. Top-view error plot of (a) a chondral defect and (b) an osteochondral defect. The laser scans of the printed surfaces were compared with the pre-damage CT scan reference geometry [47].
《Table 2》
Table 2 Properties of printed and non-printed PEGDMA with and without human chondrocytes.

a Human chondrocyte concentration in hydrogels: 5 × 106 cells·mL-1 ; w/v: weight/volume.
Although in situ bioprinting has solved the dependence on external environmental cues—such as UV light, temperature, and calcium availability—to initiate phase change after deposition for constructing tissues in vitro, there is a greater demand for precise path planning and deformation when printing in situ. Printing within a wound means that chemical modification and surface nanotopography cannot be realized by surface treatment. Researchers must therefore put more thought into preventing the bacterial adhesion of implants for in situ bioprinting.
2.3.3. Enhancing the mechanical properties of scaffolds
Personalized scaffolds, and particularly engineered cartilage substitute, should provide an environment with micro-stress that is equal to that of a natural environment for cells, maintain structure stability and integrity, and possess mechanical strength that matches those of the subchondral bone and adjacent cartilage of the implant location in order to provide an immediate and longterm load-bearing function [49]. Researchers generally adopt crosslinking technology to improve the mechanical properties of widely used gel materials due to their disadvantages of easy shrinking, brittleness, and poor mechanical properties [50]. Such technologies mainly include thermal crosslinking, ionic crosslinking, and pH crosslinking. The use of different functional groups during crosslinking prevents the use of potentially cytotoxic crosslinkers [51]. For example, Wu et al. [52] manufactured 3D printed bioactive glass scaffolds with a hierarchical pore architecture and well-ordered mesopores in various shapes, and then used polyvinyl alcohol (PVA) as a thermo-crosslinking agent to improve the mechanical properties.
The use of various crosslinking technologies can significantly improve mechanical properties; for example, ionic crosslinking can be used to deplete mechanical energy and covalent crosslinking can increase elasticity. Hong et al. [44] built an interpenetrating network to fabricate highly stretchable and tough hydrogels (Fig. 12). The toughness of the biocompatible hydrogel depends on a combination of two mechanisms: the reversible Ca2+ crosslinking of sodium alginate consumes mechanical energy, while the covalent crosslinking of PEG maintains elasticity under large deformations. Increased concentrations of polymer materials can also improve the mechanical properties of hydrogels.
《Fig.12》

Fig. 12. (a) A printed bilayer mesh is uniaxially stretched to three times its original length and almost completely restored to its original shape after relaxation; (b) a printed pyramid undergoes a compressive strain of 95% and reverts back to its original shape after relaxation [44].
Researchers have also recently developed many high-strength hydrogels with tear resistance, high cell counts, and multilayer artificial soft tissue, such as double network (DN) hydrogels [53–57]. Gong [58] found that high-strength DN hydrogels made from poly(2-acrylamido-2-methyl-1-propanesulfonic acid) (PAMPS) polyelectrolyte and polyacrylamide (PAAm) neutral polymer exhibited highly unique properties: low friction, low wear, and cell compatibility (Fig. 13(a)). Sherwood et al. [59] designed a multilayer cartilage-bone tissue-engineered composite scaffold (Fig. 13(b–e)). In the upper portion of the scaffold, the cartilage region was composed of D,L-PLGA/L-polylactic acid (PLA) to facilitate homogenous cell seeding. The lower, bone portion of the scaffold consisted of an L-PLGA/tricalcium phosphate (TCP) composite designed to maximize bone ingrowths. The transition region between these two sections contained a gradient of materials and porosity to prevent delamination. In vivo tests suggested that this scaffold had favorable mechanical properties for in vivo implantation and full joint replacement.
《Fig.13》

Fig. 13. (a) Image of a tough PAMPS/PAAm DN hydrogel; (b) cross-sectional view of an MTT-stained osteochondral scaffold after top seeding; (c) cross-sectional view of an MTT-stained osteochondral scaffold after rotational seeding; (d) outer view of an MTT-stained osteochondral scaffold after top seeding; (e) outer view of an MTT-stained osteochondral scaffold after rotational seeding [53–57]. MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Cui et al. [60] prepared double-layer polyurethane (PU)–collagen conduits for peripheral nerve repair via a double-nozzle, low-temperature fiber-deposition manufacturing system. The double-layer PU–collagen nerve conduits are shown in Fig. 14 [60]. This structural design combines the advantages of internal collagen for good cell compatibility with those of outer polymer PU for superior mechanical properties.
《Fig.14》

Fig. 14. Morphology of a double-layer PU–collagen nerve conduit. (a) Photograph of the double-layer conduit; (b) scanning electron microscope image of the interface between the two layers (cross-section); (c) microstructures of the collagen layer; (d) microstructures of the PU layer [60].
《2.4. Directly printing tissue and organs》
2.4. Directly printing tissue and organs
Encapsulating cells into biodegradable scaffolds via traditional tissue engineering cannot ensure that cells are precisely implanted into inner scaffolds, and growth factors will only affect the growth and differentiation of surface cells. Therefore, researchers have studied cell and growth factor direct-printing technology with the ultimate goal of producing tissues and organs. In 2000, Professor Thomas Boland of Clemson University, USA, proposed a new concept called ‘‘cell and organ printing” that represents the origin of modern 3D bioprinting technology. Tissue structures with physiological functions can be formed by printing various materials and ‘‘biological ink” containing seed cells, growth factors, and nutritional components layer by layer, followed by culturing the printed tissue or organ.
The biggest technical challenge of printing tissues or organs is replicating the intricate internal vascular network of organs, rather than the manufacturing process itself. Consequently, many researchers have turned their focus to blood vessel printing. In 2009, Ganovo company in the United States was the first one to use 3D printing technology to produce vascular prostheses [61]. The Southern California University of Health Sciences and the University of Michigan mixed agarose as a support with cells to co-print a vascular network less than 3 mm in size using a 3D printing device [62].
Scientists from the Wyss Institute for Biologically Inspired Engineering, Harvard University [63], reported a new 3D bioprinting method for fabricating complex living structures with integrated microvessels using multiple print heads and special "ink”. During the fabrication of tissue integrated with blood vessels, multiple types of cells, and extracellular matrix (ECM), a 3D bioprinter with several independently controlled printheads was designed in order to create these heterogeneous structures with multiple materials, which must be printed precisely and simultaneously. Gelatin methacrylate (GelMA) is used as a cell and matrix carrier, while poly(dimethyl siloxane) (PDMS) dyed with different fluorophores is used to label different biomaterials (Fig. 15(a,b)) [63]. A heterogeneous 3D architecture (Fig. 15(c)), in which each layer is composed of different GelMA, is then co-printed. Green and red fluorescent protein-expressing human neonatal dermal fibroblasts (HNDFs) and human umbilical vein endothelial cells (HUVECs) respectively, could be clearly observed (Fig. 15(d,e)), demonstrating that HUVECs can attach and proliferate in the fabricated channels to produce implantable, fully functional living tissue and even organs.
《Fig.15》

Fig. 15. (a), (b) Schematic views of our 3D bioprinting approach; (c) schematic view of an engineered tissue construct cultured; (d) fluorescence images of an engineered tissue construct cultured for 0 day; (e) fluorescence images of an engineered tissue construct cultured for 2 days. The cross-sectional view in (e) shows that endothelial cells line the lumen within the embedded 3D microvascular network [63]. HNDFs: human neonatal dermal fibroblasts; HUVECs: human umbilical vein endothelial cells.
There are successful cases of printing tissue or organs by domestic and foreign researchers. Michael et al. [64] utilized a laser-assisted bioprinting technique to create a fully cellularized skin substitute by placing fibroblasts and keratinocytes in an exact 3D spatial pattern (Fig. 16). The printed skin construct, consisting of fibroblasts labeled in red and keratinocytes labeled in green on top of MatriDerm®, is placed into the wound in the mouse skin (Fig. 16(b)). The other parts of the mouse skin remain intact as a contrast. Upon testing the skin constructs by employing the dorsal skin fold chamber in nude mice, the printed cells remained active, continued proliferating, and secreted ECM. Furthermore, as shown in Fig. 16(c), some blood vessels were found growing from the wound bed and the wound edges. A bioprinting technique for multiple layers of cells is a prerequisite for creating more complex tissue.
《Fig.16》

Fig. 16. (a) Scheme of the 3D special pattern made of fibroblasts and keratinocytes in mice; (b) tissue-engineered skin construct inserted into a wound in the dorsal skin fold chamber in nude mice directly after implantation; (c) tissue-engineered skin construct inserted into a wound in the dorsal skin fold chamber in nude mice on day 11 after implantation [64].
Mannoor et al. [65] generated a bionic ear by 3D printing a chondrocyte-seeded alginate hydrogel matrix and infused silver nanoparticles in the anatomic geometry of a human ear and cochlea-shaped electrodes (Fig. 17(a,b)). The printed bionic ear possessed better auditory sensing of radio frequencies than the human ear. A biologist from Cornell University utilized stem cells and biopolymer materials to print a functioning cardiac valve (Fig. 17(c)), and the stem cells gradually differentiated into human cells. There currently exist some 3D printed organs for clinical applications. As shown in Fig. 17(d), researchers at the University of Michigan implanted a 3D printed artificial trachea into the windpipe of an infant with a birth defect to assist breathing, representing the world’s first successful 3D printed human organ transplant.
《Fig.17》
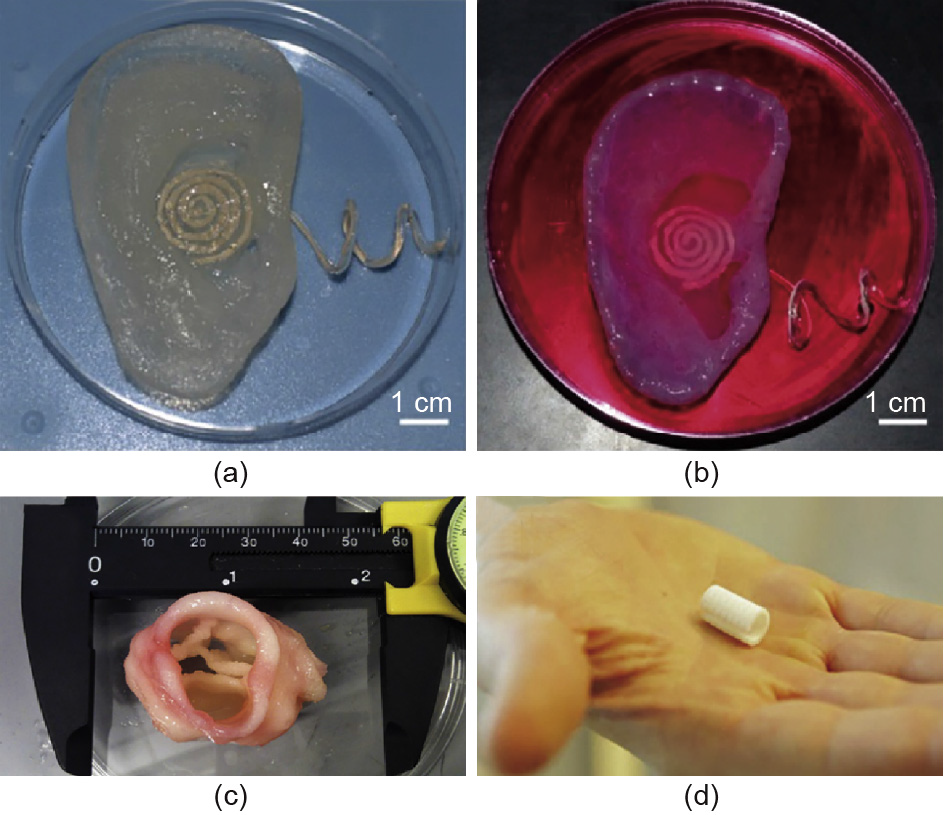
Fig. 17. (a) Image of the 3D printed bionic ear immediately after printing; (b) image of the 3D printed bionic ear during in vitro culture; (c) the 3D printed cardiac valve; (d) the 3D printed artificial trachea [65].
《3. Biomedical material in 3D printing 》
3. Biomedical material in 3D printing
Biomedical materials (Fig. 18), including hard metals (Ti), polymers (from thermoplastic polymers (polystyrene and PLGA), to elastomeric polymers (PDMS), to soft hydrogels), and ceramic materials (HA) have been broadly used to create permanent implants or adopted as matrix substrates by 3D printing [66].
《Fig.18》

Fig. 18. 3D printed parts from various materials. (a) Ti alloy prosthesis; (b) polymer plate made from new resorbable ceramic biomaterials; (c) collagen [78] gel scaffold; (d) HA [79] scaffolds.
《3.1. Medical metal materials 》
3.1. Medical metal materials
Medical metal materials are mainly applied to prepare permanent implants such as orthopedic or dental implants containing stainless steel, cobalt-chromium alloy, Ti alloy (Fig. 18(a)), or tantalum alloy. These compositions of metal materials possess good biocompatibility that meets medical standards.
3D printed biomedical metal materials have advantages over traditional implants. 3D printed metal implants tend to have small grains with better mechanical performance. In addition, highly controlled printing environments ensure high purity of the printed parts, thus maintaining the desired characteristics of the material. Moreover, the design complexity of 3D printed personalized products is reduced, allowing customization of implants with mechanical behaviors that are similar to those of bone or teeth. Surface treatment, such as electrochemical deposition, chemical modification, and alkali-heat treatment, is usually carried out to enhance the bioactivity of porous metal implants. As part of the metal 3D printing value chain, heat treatment can tailor biocompatibility and mechanical properties simultaneously. The surface of SLM fabricated Ti6Al4V is more hydrophilic, rougher, and more homogeneous after a suitable heat-treatment process and parameters. Surface feature analysis indicated that the modulus and hardness were more stable in resisting cracking and fatigue, while cell proliferation analysis revealed enhanced cell adhesion and uniform distribution, owing to optimized biocompatibility after heat treatment [67]. Thus, creating customized implants by 3D printing metal material with subsequent heat treatment is beneficial for obtaining desirable physiochemical properties and avoiding extra cost in improving cytocompatibility.
Many metal materials are being developed for medical application because of the cytotoxic Al or V in common Ti6Al4V porous scaffolds. For example, Ti-Nb alloys are more effective for biomedical applications, due to their very low elastic modulus, excellent biocompatibility, high strength, and low cytotoxic element content [68]. To further reduce the elastic modulus in order to minimize the adverse impact of stress shielding, tantalum (Ta) can be added as a stabilizing element in Ti [69]. Ti-Ta bulks that are made via SLM exhibit a higher strength and lower elastic modulus than Ti6Al4V. Sing et al. [70] even proved the feasibility of lasermanufactured porous Ti-Ta cellular lattice structures based on the regression analysis method. Process parameters greatly influence the dimensional accuracy and mechanical properties of Ti-Ta alloy lattice structures. Researchers can also use SLM technology to solve the problem of processing intricate structures while maintaining the functional properties of Ni-Ti alloys, which possess superelasticity and shape memory [71]. Special Ni-Ti unit cells exhibit compression properties within the range of those of cortical and trabecular bone, and show improved fatigue life. Magnesium (Mg) also has great potential for implants due to its low corrosion potential to completely degrade in the body and to its Young’s modulus, which is similar to that of natural bone Mg and thus reduces the stress-shielding effect [72]. Mg is one of the essential components in the human body to promote the proliferation and differentiation of bone cells.
Some novel antibacterial alloys with viable mechanical properties and biocompatibility, such as CoCrWCu [73], have been further developed to solve the problem of bacterial infection or inflammation, since Cu exhibits well-known antimicrobial activity and promotes metabolism. Lu et al. [74] investigated the influence of Cu on CoCr-based alloys, which are widely used in orthopedics and dentistry. CoCrW was verified to be non-cytotoxic, and allowed cells to adhere and proliferate on the surface well (Fig. 19(a,c)) during the cell viability test. Antibacterial testing confirmed the excellent antibacterial performance against E. coli that resulted from the addition of a certain amount of Cu (Fig. 19(b,d)).
《Fig.19》

Fig. 19. (a) Fluorescence microscope images of MG63 cells with CoCrW; (b) E. colibacterial colonies cultured on CoCrW alloy; (c) fluorescence microscope images of MG63 cells with CoCrW-3Cu; (d) E. coli bacterial colonies cultured on CoCrW-3Cualloy.
《3.2. Medical polymer materials 》
3.2. Medical polymer materials
Polymer materials consisting of natural biomaterials and synthetic biomaterials are primarily used to prepare medical models and biodegradable scaffolds. Chitosan, collagen (Fig. 18(c)), and fibrin are widely used natural medical polymeric materials. These polymers possess excellent compatibility, promote cell adhesion and proliferation, and maintain cell phenotypes, although they can easily deform, resulting in poor mechanical strength. The shape, relative molecular mass, and degradation time of synthetic polymer materials such as PLA, PVA, and polycaprolactone (PCL) [75] can be precisely controlled. However, the surfaces of polymers lack recognition sites for cell adhesion, leading to heterogeneous cell distribution and cell loss. Therefore, the mechanical performance, fluidity, and surface roughness of polymers must be enhanced in order for them to be of use in printing processes and medical implants.
Yue et al. [76] integrated more complex functions into polymers. After preparing antimicrobial composite resins, they found that antibacterial 3D printed implants killed bacteria on contact without damaging human cells, and could therefore eventually be used to replace traditional dental fillings. Moreover, the method used for 3D printing antimicrobial polymers can easily be transferred to other nonmedical application, such as food packaging, water purification, or even manufacturing toys for children.
《3.3. Medical ceramic materials 》
3.3. Medical ceramic materials
Ceramic materials (mainly HA and TCP) are widely used for artificial joints or dental implants because of their stable physicochemical characteristics, high biocompatibility, and osteoconductivity [77]. They are also ideal dental materials due to their controllable shapes and sizes, and can be easily colored during 3D printing. HA (Fig. 18(d)), which is a type of ceramic material and a key mineral component in bone and tooth tissue, is suitable for rapid prototyping such as selective laser sintering (SLS) and SLM in clinical applications. New resorbable ceramic biocomposites (Fig. 18(b)) developed by the EU’s project titled RESTORATION (i.e., Resorbable Ceramic Biocomposites for Orthopedic and Maxillofacial Applications) will be available for orthopedic and maxillofacial applications soon. Implanting 3D printed custom joint prostheses will greatly reduce patient pain while allowing minimally invasive surgery.
Compared with metals and polymers, most ceramics have a different consolidation mechanism and obvious residual stresses after sintering, which could influence the mechanical strength and pore morphologies. During the SLM or SLS process, the powder layer deposition, laser–powder interaction, thermal and residual stress, and so on are crucial factors in the fabrication of fully dense ceramic [80]. The toxicity of residual binders will be also tested in cells in vitro. Future research on fully dense ceramics should focus on Fig. 18. 3D printed parts from various materials. (a) Ti alloy prosthesis; (b) polymer plate made from new resorbable ceramic biomaterials; (c) collagen [78] gel scaffold; (d) HA [79] scaffolds. Fig. 19. (a) Fluorescence microscope images of MG63 cells with CoCrW; (b) E. coli bacterial colonies cultured on CoCrW alloy; (c) fluorescence microscope images of MG63 cells with CoCrW-3Cu; (d) E. coli bacterial colonies cultured on CoCrW-3Cu alloy. Q. Yan et al. / Engineering 4 (2018) 729–742 739 enhancing their biomechanical properties, printing resolution, biocompatibility, and sustained drug release [81].
The biomaterials described above have both advantages and disadvantages regarding their use in clinical application; therefore, implants usually integrate different materials in order to achieve multiple functions [82]. For example, three types of biomaterials (i.e., metal, polymer, and ceramic) can be used to fabricate porous scaffolds to meet the implant requirements. Researchers have combined HA-coated porous Ti as a carrier with BMP-2 via gelatin in order to successfully prepare a 3D porous active composite material [15].
Advancement in materials will accelerate the development of 3D printing in biomedical applications. In addition to existing traditional materials, shape-memory materials (SMMs), also known as smart materials, are attracting a considerable amount of attention. SMMs with reversibility can switch their shape or properties in a predefined manner under specific external stimulus [83]. A customized implant is transplanted into the wound, and then transforms its shape when the temperature, pressure, or magnetic field changes, in order to adapt to the dimensions of tissue defects. Thus, 3D printed smart materials show great potential for minimally invasive therapy in biomedical applications.
《4. Limitations 》
4. Limitations
The use of 3D printing technology for medical applications will efficiently solve the donor-shortage issue for organ transplantations, and is therefore an emerging and rapidly developing interdisciplinary field that tightly integrates material science, biology, and clinical science. Although cells can be directly printed at this stage, much work remains to be done in order to achieve the goal of the in vitro engineering of tissue [84]. It is difficult to simulate the structure and biological function of the ECM in vitro, as it is a complex system with multiple components. Existing technologies that primarily stack cell-seeded hydrogels are unable to solve the issues of cellular nutrition and oxygen supply. For larger scaffolds, a suffi- cient number of cells cannot be provided at present. Compared with cells that attach to the surface of scaffolds, preprophase cells do not receive an adequate supply of nutrients. That is, the cells survive in disequilibrium in the 3D space [85]. In addition, other limitations that include cell survival, development, differentiation, and fusion, must be solved for the further development of printed scaffolds, tissues, and organs.
Limitations also exist in terms of materials. Many of the metal materials that are generally used for permanent implants have a high elastic modulus, which often leads to an elastic mismatch between the implant and the bone. Printed biodegradable scaffolds are generally fabricated from natural polymers with good biocompatibility but poor mechanical properties, such as collagen, sodium alginate, and other hydrogels. In addition, international standards for choosing medical materials for 3D printing have not been developed; thus, only synthetic evaluations can be made based on structure, function, clinical effects, and other aspects, rather than evaluations based on reliable indicators and sufficient experimental evidence.
3D printing was originally used in engineering rather than medicine, although medical-oriented 3D printing techniques have been rapidly developed in recent years, requiring multifaceted knowledge. At present, medical and engineering researchers have relatively independent research fields and structural frameworks, which has partially limited the development of this technology for medicine. Cell induction and ethical issues should not be neglected. Thus, achieving the actual implementation of 3D printing for medical applications will require long-term efforts [86–88].
《5. Conclusions and future directions》
5. Conclusions and future directions
Great progress has been made in the field of medical-oriented 3D printing technology, and the manufacturing technology of organ models and permanent implants has become more mature. Researchers have successfully used various methods to enhance the mechanical behavior of personalized biodegradable scaffolds. Although the direct printing of tissues and organs is still in the initial stages, domestic and international researchers using printed tissue and organs have begun to study the printing of vessels. Medical biomaterials used in 3D printing consist of metals, polymers, and ceramics, with multiple materials usually being integrated in order to achieve complex functions in the printed components. Although 3D printing has already been realized in clinical applications, 3D printing technology is still limited in terms of materials and in the construction of ECM in vitro. Much work remains to be done before printed bioactive tissues and organs can truly be applied in the clinic.
In the future, researchers will solve these problems to successfully integrate 3D printing with tissue engineering. Future work includes developing new equipment to guarantee the high porosity and dimensional precision of scaffolds; studying high-performance materials for various medical-oriented 3D printing techniques; creating unified standards for 3D printed scaffolds; strengthening market supervision in order to optimize implants for clinical use; and establishing a 3D printing platform in order to enhance communication among hospitals, companies, and research institutes. These advancements will further promote the development of 3D printed tissue engineering scaffolds.
《Acknowledgements》
Acknowledgements
This work was sponsored by the National Key R&D Program of China (2016YFB1101303), the Wuhan Morning Light Plan of Youth Science and Technology (0216110066), and the Academic Frontier Youth Team at Huazhong University of Science and Technology.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Qian Yan, Hanhua Dong, Jin Su, Jianhua Han, Bo Song, Qingsong Wei, and Yusheng Shi declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




