《1. Introduction》
1. Introduction
Traditional Chinese medicines (TCMs), also known as herbal medicines or phytomedicines, have a long history of preventing diseases, alleviating symptoms, and improving health. These medicines include seeds, roots, leaves, rhizomes, bark, and flowers [1]. The Shennong Bencao Jing (literally the Shennong’s Classic of Materia Medica) and the Huangdi Neijing (literally the Inner Canon of Huangdi Neijing) are good examples of historical collections of TCM formulas. In the past, researchers have tried to elucidate the mechanisms of action of TCMs, and have focused on the bioactive compounds in TCMs. However, most chemicals from TCMs have been shown to have little or even no bioactivity or bioavailability [2,3]. Therefore, research to date has been unable to explain the mechanisms of TCMs actions. New research insight is needed in order to learn more about the mechanisms of action of TCMs. Recent research has focused on a previously overlooked target: the gut microbiota.
As an important part of the body, the gut microbiota behaves as an additional organ in many ways [4]. In terms of “holistic medicine” within Chinese medicine, gut microbes are obviously significant to TCMs. Several studies have implicated the role of the gut microbiota in many human diseases (Fig. 1), including nonalcoholic fatty liver disease (NAFLD) [5], inflammatory bowel disease (IBD) [6], obesity [7], diabetes [8,9], irritable bowel syndrome (IBS) [10], and cancer [11]. In addition, many interactions between the chemical components of TCMs and the gut microbiota are currently under further study [12,13]. To be specific, the gut microbiota can metabolize TCM chemicals alone or co-metabolize chemicals with the host; the generated metabolites possess varying degrees of bioavailability, bioactivity, and toxicity. The composition of the gut microbiota for homeostasis recovery can also be modulated beneficially by TCM components. Thus, TCMs can ameliorate dysfunction of the gut microbiota along with relevant pathological conditions [1]. Based on the above findings, the gut microbiota can mediate either synergistic or antagonistic effects among multiple TCM components [1]. Therefore, we believe that the gut microbiota plays an important role in the therapeutic effects of TCMs. This review may provide new insight into current understanding of how TCMs work.
《Fig. 1》

Fig. 1. The effect of TCMs on some organs through the gut microbiota.
Here, we focus on summarizing the present understanding of the role of gut microbes in the activity of TCMs. First, we briefly introduce the interplay between the gut microbiota and host health. Next, we discuss the relationship between TCMs and certain diseases, as well as the corresponding mechanisms associated with the gut microbiota. Finally, we provide our perspective on further relevant research.
《2. Gut microbiota and host health》
2. Gut microbiota and host health
In humans, more than 100 trillion microbes live in the oralgastrointestinal tract (GIT). Most of these microbes reside in the gut, where the highest density of all microbial habitats has been recorded [14]. The gut microbiota co-develops with the host, and the total genome of the human gut microbiota alone contains approximately three million genes, which is 100 times more than the number of genes encoded by its mammalian host [15,16]. Some gut microbes can provide various signals for intestinal development, including angiogenesis, mucosal barrier fortification, and postnatal intestinal maturation [17]. It is notable that gut microbes interrelate with the host immune system, prompting immune cells to produce cytokines, which can affect neurophysiology [18], as well as providing signals to help immune cells mature and immune functions develop normally [4].
Here, we briefly review the current understanding of the effect of the gut microbiota on host health and diseases, and the pathways involved. In general, the microbiota impacts the host via several axes—the gut–liver axis, gut–brain axis, gut–muscle axis, or in situ gut—and functions via the following four principle physiological pathways as follows [1]:
(1) Participation in host metabolism. The gut microbiome possesses a much more powerful metabolic capability than the host genome due to the presence of a far greater number of metabolic enzymes. Many substrates are co-metabolized with the host, or even metabolized independently by the gut microbiota [19].
(2) Formation of global immunity. Host congenital and adaptive immune responses both involve the intestinal flora, which has been confirmed by immunological defects and subsequent pathological improvements after intestinal flora transplantation in germ-free models [20]. Further research has revealed that the mechanisms mentioned above are associated with cellular immunity and lymphoid organogenesis [21–23].
(3) Homeostatic maintenance of the GIT. In addition to global immunity, the gut microbiota participates in mucosal immunity to maintain a balanced intestinal ecosystem. In order to protect itself, the intestinal flora prevents the occurrence of an excessive immune response by inducing the differentiation of resident T cells into T helper 2 and regulatory T (Treg) subpopulations [24]. In addition, the gut microbiota can prompt the production of immunoglobulin A (IgA), which inhibits overgrowth of the bacterial population [25]. Gut microbes assist with intestinal homeostasis via competitive exclusion and the induction of antimicrobial substances that act against invading pathogens [26]. In addition, the gut microbiota facilitates GIT structural maturation by promoting angiogenesis [27], gut barrier maintenance [28], and mucosal glycosylation [29].
(4) Effects on brain function and host behavior. The gut microbiota interacts with the central nervous system via the brain–gut axis, which involves neural, endocrine, and immune pathways; thus, it impacts both brain function and host behavior [30]. In addition, the gut microbiota can affect brain function and behavior by regulating tryptophan metabolism [31] and producing neuroactive metabolites [32] and neurometabolites.
Most TCMs are used for chronic treatment and are administered orally [33]; this results in sufficient opportunities for eventual “intimate” exposure to the entire gut microbiota. There is evidence to suggest that herbs and other dietary components strongly interact with intestinal microbes and impact human health [34–37], and an increasing quantity of evidence indicates that the gut microbiota plays a key role in TCM therapy via complex interactions with TCM components. These interactions include the following activities: The intestinal microbiota biotransforms TCM components into metabolites with different bioavailabilities and bioactivities/ toxicities than their precursors [12,13]; TCM chemicals improve the composition of the gut microbiota, thereby alleviating dysfunction of the gut microbiota and related pathological conditions; and the intestinal microbiota mediates the interactions (synergy and antagonism) between various chemicals in TCMs.
Interestingly, some diseases can be ameliorated by changes in the gut microbiota that favor homeostasis [4,38,39]. Although the exact mechanisms underlying the ecological imbalance and pathology of the intestinal microbiota are not yet understood, these associations have been clearly confirmed.
《3. TCMs, gut microbiota, and diseases》
3. TCMs, gut microbiota, and diseases
《3.1. TCMs, the gut microbiota, and chronic liver diseases》
3.1. TCMs, the gut microbiota, and chronic liver diseases
Chronic liver diseases include infectious liver disease, metabolic liver disease, and autoimmune liver disease, all of which can have the same final outcome—cirrhosis and liver cancer. There is sufficient evidence to show that patients with chronic liver diseases have significant imbalances in their gut microbiota: namely, decreased abundance of beneficial bacteria and increased abundance of harmful bacteria [40–44]. A large number of patients in China suffer from liver diseases, and TCMs have been successfully used to treat these diseases for thousands of years.
Berberine, an isoquinoline alkaloid that is most produced by Chinese medicine plants like Coptis chinensis (i.e., Rhizoma coptidis), has been shown to cause remittance of NAFLD, with berberine treatment leading to the abovementioned metabolic improvement and increased abundance of Bacteroidetes, Bacteroidetes/Firmicutes, Bifidobacterium, and Lactobacillus [45]. An ancient Chinese herbal formula, Qushi Huayu Fang, exhibits a similar effect. This formula alleviates hepatic steatosis, decreases triglyceride content and free fatty acid levels in the liver, and modulates the gut microbiome, especially the genera Escherichia/ Shigella and Collinsella [46]. Dong et al. [47] introduced and compared 21 TCMs for their potential benefits in the treatment of NAFLD, and found that most of the TCMs exhibited improved biochemical and histological changes associated with NAFLD, both in vitro and in vivo.
The Yinchenhao decoction is the main traditional prescription for the treatment of jaundice. The preparation of this decoction is described in the Shanghan Lun (literally the Treatise on Cold Pathogenic Diseases) written by Zhang Zhongjing from the Eastern Han Dynasty, and mainly consists of the herbs capillaris, medlar, and rhubarb. Studies have suggested that the Yinchenhao decoction can inhibit liver injury, liver cell apoptosis, hepatic stellate cell (HSC) activation, and collagen synthesis and promote bilirubin metabolism [48–52]. Fuzheng Huayu Fang is another ancient formula that is mainly used to modulate fibrosis [53]. Silymarin is a novel flavonoid extracted from the seeds of Silybum marianum L. Gaertn. One of the main components of silymarin is silychristin, which has been shown to play a complex role in antiinflammation and immunomodulation, and which has hypolipidemic, antioxidant, and hepatoprotective effects [54–57]. Due to the evidence above, silymarin-based therapy is currently recommended for liver diseases and is even included in several Chinese guidelines.
TCMs are mostly multicomponent macromolecules. These molecules have extremely complex roles and effects in liver diseases and are associated with physiological barriers, metabolism, and immune inflammation [47]. Herbal monomers and active substances have been found to have hepatoprotective activities, in an investigation of the hepatoprotective activity of Zhizidahuang decoction, Yinchenhao decoction, and Dahuangxiaoshi decoction [58]. Studies have shown that TCMs and the gut microbiome interact closely. The gut microbiota can catabolize macromolecular substances in TCMs into bioactive polyphenols, alkaloids, and other active monomers, and TCMs can regulate the structure of the gut microbiome [12,13].
Studies have revealed that the therapeutic mechanisms of berberine are involved in the significant reduction in cluster of differentiation 14 (CD14), Interleukin 1 (IL-1), Interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α) levels in hepatic tissue [1,45]. In addition, anti-TNF-α antibodies can alleviate NAFLD, which leads to an amelioration in liver steatosis and a reduction in total fatty acid levels in liver tissue and alanine aminotransferase levels in serum [59]. In the gut microbiota, Bifidobacterium and Lactobacillus can inhibit the disruption and stimulation of TNF-α [60]. Furthermore, a probiotic (VSL#3) exhibited a similar effect to that of berberine [59]. This finding may therefore indicate that probiotics participate in the effect exerted by berberine. One study found that 6,7-dimethylesculetin, an active compound in virgate wormwood herb, activates the constitutive androstane receptor and accelerates bilirubin clearance in vivo [52]. Genipin, a gut bacterial metabolite of geniposide, which is a major ingredient of the Yinchenhao decoction, may prevent apoptosis by interfering with mitochondrial permeability transition and HSC inhibition via the inhibition of DNA synthesis to elicit therapeutic effects [49,61]. Silymarin is an active component with anti-lipid peroxidation activity; the antioxidant activity of this compound may occur via inhibition of the release of superoxide anions from neutrophils and increased expression of superoxide dismutase in lymphocytes [62–64]. Moreover, silymarin inhibits the activity of inhibitor of nuclear factor kappa-B kinase subunit beta (IKK-β), p50, and p65, thereby inhibiting the effects of 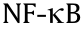 and achieving antiinflammatory and hepatoprotective effects [65,66] (Table 1 and Fig. 2).
and achieving antiinflammatory and hepatoprotective effects [65,66] (Table 1 and Fig. 2).
《Table 1》
Table 1 Relationships between TCMs and diseases in terms of the gut microbiota.
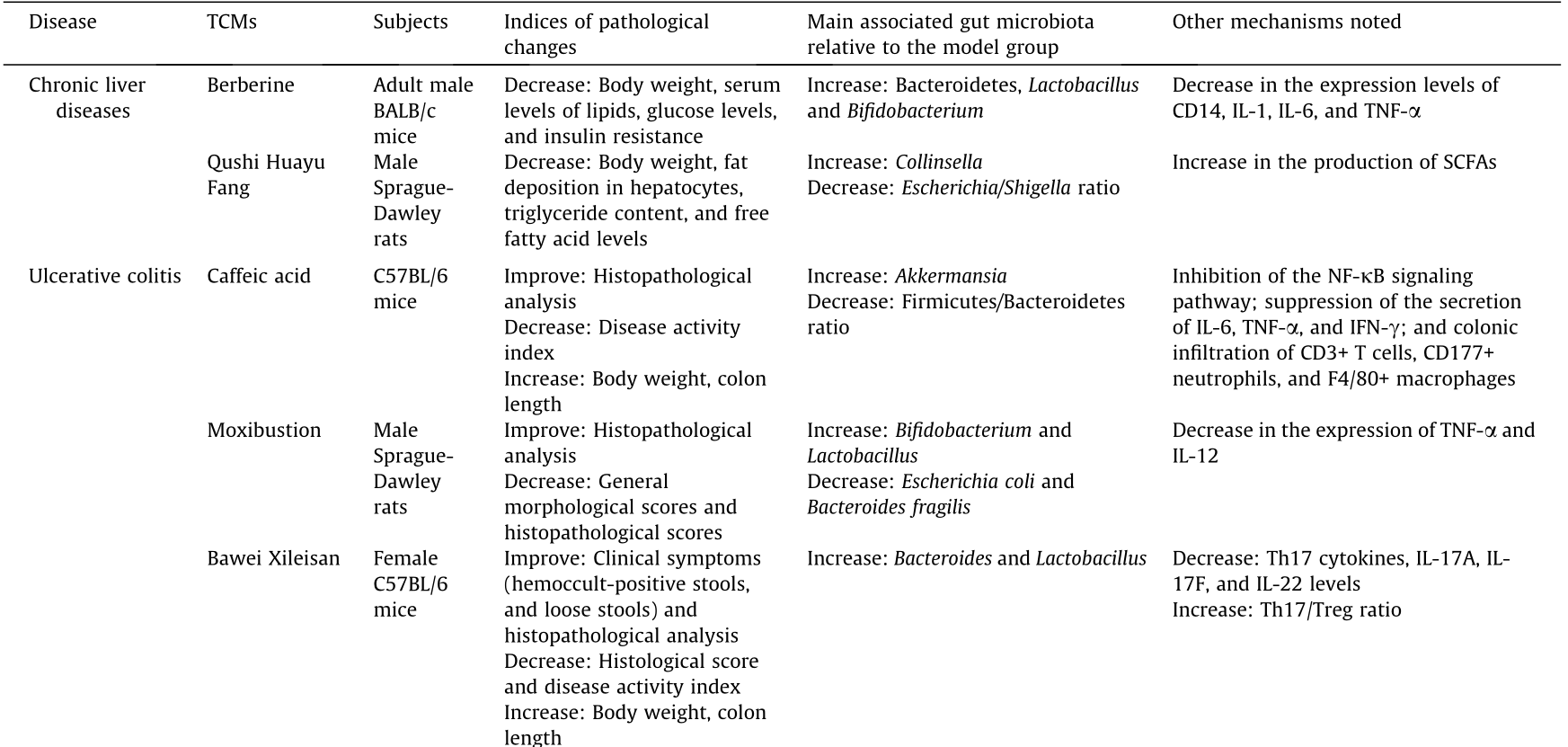


Table 1 (continued)
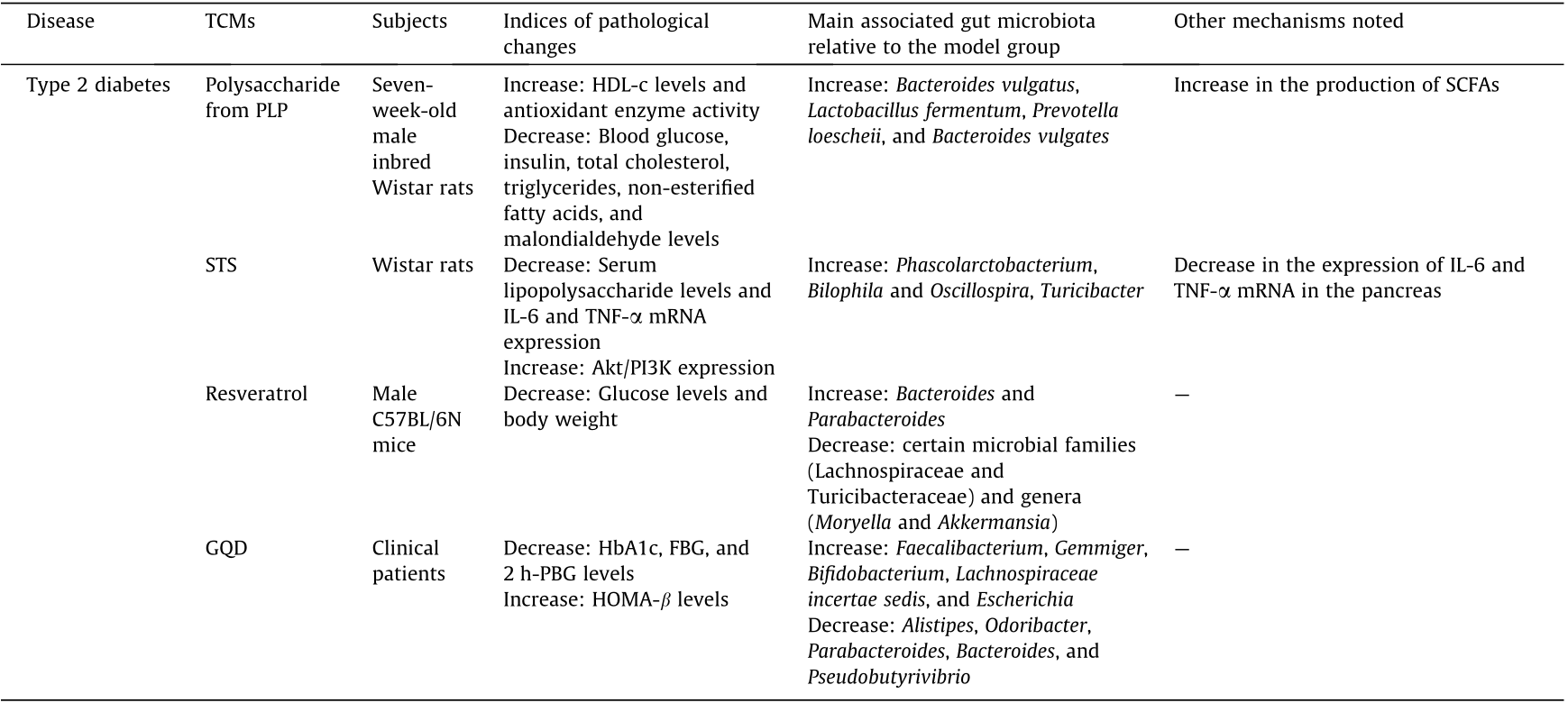
PLP: Plantago asiatica L.; STS: stachyose; GQD: Gegen Qinlian decoction, with major components of flavones, alkaloids, and triterpenoid saponins; SCFAs: short-chain fatty acids; IFN-γ: interferon-γ; Th17: T helper 17 cells; PGE(2): prostaglandin E(2); iNOS: inducible nitric oxide synthase; PTGES: prostaglandin E synthase; HOMA-β: homeostasis model assessment β; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; HDL-c: high-density lipoprotein cholesterol; HbA1c: glycosylated hemoglobin, hemoglobin A1c; FBG: fasting blood glucose; 2 h-PBG: 2 h postprandial blood glucose.
《Fig. 2》
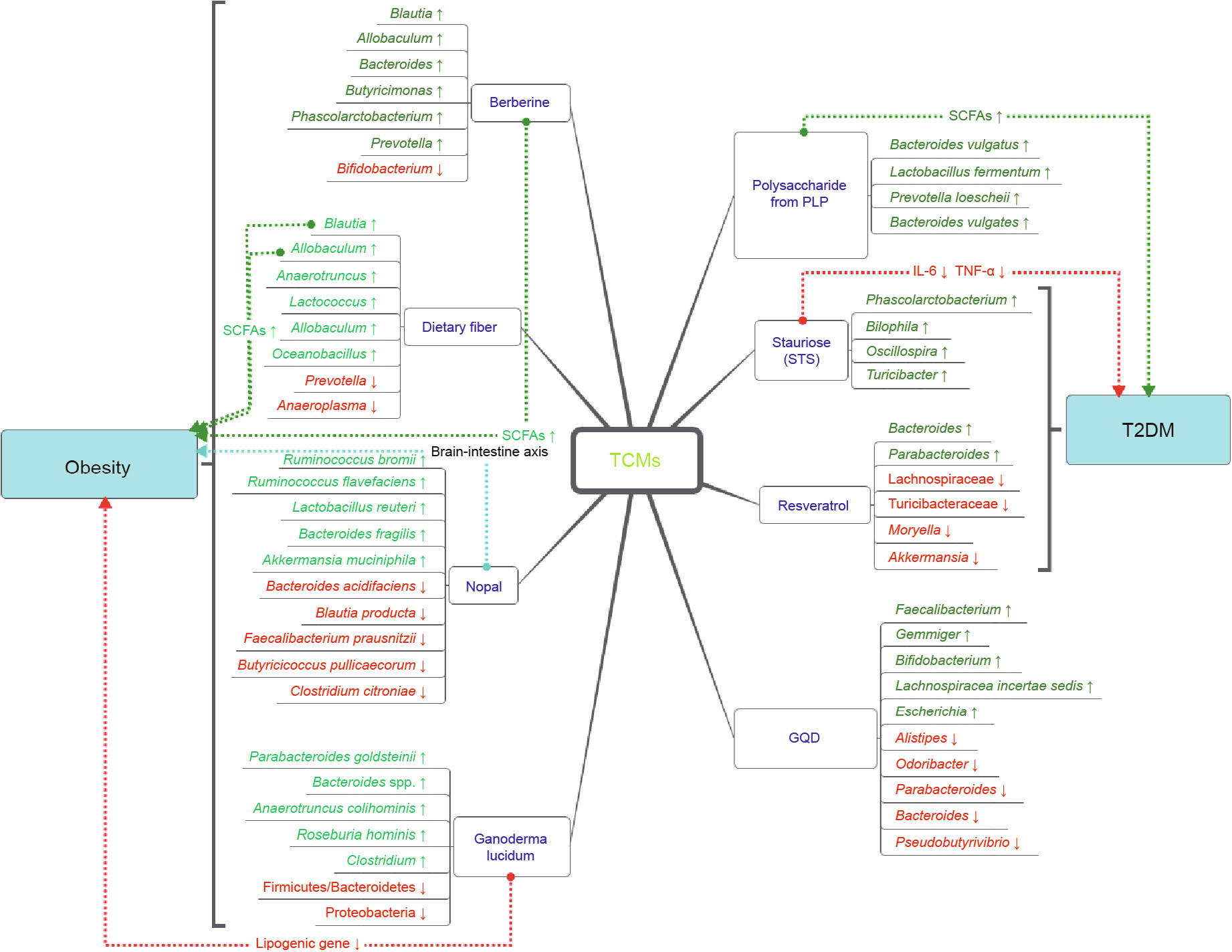
Fig. 2. Changes and potential mechanisms of the major gut microbiota of ulcerative colitis and chronic liver disease when treated by TCMs. Blue font represents TCM, green font shows increased flora in the experiment, and red font shows decreased flora. The dotted arrow indicates the direction of action, and the text on the dotted line represents the underlying mechanism. Blue dotted lines indicate other potential mechanisms of action. T2DM: type 2 diabetes.
《3.2. TCMs, the gut microbiota, and ulcerative colitis》
3.2. TCMs, the gut microbiota, and ulcerative colitis
Ulcerative colitis (UC) is a disease that is clinically characterized by inflammation and ulcers in the colon and rectum. Although the cause of UC remains unknown, hypotheses regarding the pathogenesis of this disease include environmental factors, genetics, immunodeficiency, and changes in the intestinal microbial spectrum [67]. There are several classical medications that are used to treat this disease, such as sulfasalazine and steroids. In addition, biological therapy is becoming increasingly popular, including fecal bacterial transplantation. This treatment is based on certain hypotheses, including one by Ghouri et al. [68], who found that the extended time of relapse for UC is associated with intestinal gram-positive bacteria.
According to published studies, various types of TCMs can improve both the clinical symptoms and pathological results of UC in rat models and humans. Resveratrol, which is present in high levels in fruits, has been shown in two clinical cases to partially improve inflammation in humans with UC [69], while caffeic acid, which is also a dietary component, exhibited anti-inflammatory effects in mice with colitis [70]. In a study using a rat model, 40 male Sprague-Dawley rats were randomly divided into the following four groups: normal (sham), model (UC), herb-partition moxibustion (HPM-treated), and positive control sulfasalazine (SAtreated). HPM treatment used moxa cones, which were placed within a medicinal formula composed of radix aconite, cortex, radix, carthami, and salvia miltiorrhizae. The results showed that the HPM-treated group exhibited significantly lower general morphological and immunopathological scores than the model group [71]. Other articles have also indicated that familiar TCMs, such as Bawei Xileisan (composed of watermelon frost, calcite, cow gallstone, pearl powder, borax, borneol, ammonium chloride, and qing-dai) [72], red ginseng, Semen Coicis [37], and nano astragalus, have potential effects in the treatment of UC. A previous review of IBD treatment reported that wheatgrass juice powder, herba andrographitis, thunder god vine, and 23 other TCMs seem to be effective against IBD, but a lack of supervision and standards, high costs, side effects, and toxicity have restricted the application of TCMs [73].
As mentioned above, many TCMs exhibit poor absorption in bioavailability studies; therefore, the mechanism of action of these compounds in the treatment of UC remains unknown. Marchesi et al. [74] studied the excrement of patients with IBD (including UC and Crohn’s disease) and found that the co-metabolite levels of the intestinal flora (acetic acid, butyric acid, methylamine, and trimethylamine) all decreased, demonstrating that the fecal metabolic spectrum of patients with IBD differed from that of normal people. Furthermore, UC and Crohn’s disease can be distinguished by their fecal metabolic spectra [74]. A previous review including 21 randomized controlled trials (RCTs) of patients with UC treated with probiotics, prebiotics, and/or synbiotics showed that compared with conventional treatment groups, patients treated with probiotics exhibited better results, including improvement in the overall induction of remission [68]. Therefore, the beneficial effects of TCMs in UC patients and rats may be dependent on the gut microbiota.
Resveratrol can be cleaved into glycoside ligands by cytoplasmic glucosidase after being absorbed as a type of glycoside. Animal studies have shown that resveratrol can reduce pro-inflammatory cytokine levels, intercellular adhesion molecule/vascular cell adhesion molecule (ICAM/VCAM) expression, neutrophil infiltration and oxidative stress and suppress the TLR4/NF-kB pathway, thus increasing the expression of SIRT1 and enhancing the antioxidant effect; however, the β-glucosidase produced by the intestinal flora can also degrade resveratrol [69]. Caffeic acid restores the richness of the intestinal flora and inhibits the increase in the ratio of Firmicutes to Bacteroidetes. Aldehydes significantly increase the abundance of Akkermansia bacteria, which degrade mucoproteins [70]. Consistent with this finding, compared with a model group, populations of Bifidobacterium and Lactobacillus, which are well-known beneficial bacteria, significantly increased in HPM and SA groups, while populations of Escherichia coli and Bacteroides fragilis, which are well-known harmful bacteria associated with colon disease, decreased. Furthermore, the expression of TNF-α and IL-12, which are associated with inflammation, was lower after HPM and SA treatment than in the UC model. However, the internal connection between the microbiota and inflammatory factors has not been further studied [71]. In a rat model of colitis, ellagitannin-rich pomegranate extract significantly increased the populations of Bifidobacterium and Lactobacillus spp., as did urolithin-A, the main metabolite of ellagitannins. Interestingly, the anti-inflammatory properties of urolithin-A are much stronger than those of the parent ellagitannin-rich extract [75]. Regarding the potential mechanism of Bawei Xileisan, it was reported that Bawei Xileisan treatment groups exhibit increased counts of Bacteroides, which are acknowledged to be among the most effective bacteria to ameliorate UC-associated edema and mucosal tolerance, relative to the control group [72]. Red ginseng and Semen Coicis exhibit the ability to enhance the in vitro growth of Bifidobacterium and Lactobacillus, which are known probiotics, while red ginseng also inhibits the growth of several different strains of pathogens, thereby resulting in the alleviation of UC symptoms [37]. The nanopharmaceutical astragalus substantially readjusts the intestinal microecological imbalance; 7 d of treatment with this compound significantly increased the abundance of Bifidobacterium and Lactobacillus in rat intestines. Meanwhile, the counts of Enterococcus and Escherichia coli and the intestinal flora ratio decreased to normal levels. Volatile fatty acid content in the colon increased, and bacterial translocation in the liver was effectively controlled. In conclusion, after these abovementioned treatments, the gut microbiota exhibited some changes. Therefore, as the gut microbiota may play at least a partial important role in the potential mechanism of action of these medicines, these mechanisms should be investigated further in future research (Table 1 and Fig. 2).
《3.3. TCMs, the gut microbiota, and obesity》
3.3. TCMs, the gut microbiota, and obesity
Obesity is a worldwide epidemic metabolic disease that is associated with a low-grade systemic and chronic inflammatory condition [76]. In the past decade, causality of obesity has been proved to involve the gut microbiome [77,78].
Over decades of practice, TCMs have been found to have favorable effects in the treatment of obesity. Berberine is a typical example. Berberine is the main active component of Rhizoma coptidis, which has been used for centuries as an antipyretic and as an antidote against poisons in Chinese medicine [79]. Recent studies have shown that berberine acts extensively on metabolism and can effectively improve the levels of serum indices, such as serum lipopolysaccharide-binding protein, monocyte chemoattractant protein-1, adiponectin, pyruvic acid, leptin, serotonin, and ketogenic and glycogenic amino acids [35,80]. Furthermore, metabolic improvement of hypotaurine and methionine in the liver; pyridoxine and 4-pyridoxic acid in the urine; and putrescine, deoxycholate and lithocholate in the feces has also been observed [80]. These metabolic changes may be associated with obesity to varying degrees. Berberine-treated rats exhibit significant disorder in the gut microbiota and selectively enrich a few short-chain fatty acid (SCFA)-producing bacteria [35,81].
Dietary fiber is a main ingredient of TCMs. Dietary fibers such as bamboo shoot fiber and bitter melon may prevent high-fat dietinduced obesity by modulating the gut microbiome [82,83]. As a vegetable rich in dietary fiber, prickly pear has a similar effect [84]. In addition, nopal is rich in polyphenols, which constitute a well-known group of heterogeneous secondary metabolites that are found in many medicinal plants and food ingredients. Accumulating evidence has shown that a diet rich in polyphenols and plant polysaccharides can modulate the gut microbiota and may have an impact on diabetes and obesity; the gut microbiota also participates in the extensive metabolism of polyphenols, which can in turn modulate the biological activities of the gut microbiota [85].
Berberine is known to have poor oral bioavailability, so modulation of the gut microbiota has been hypothesized to be one of the mechanisms [86,87]. Berberine treatment can enrich SCFAproducing bacteria, and the effects of SCFAs may improve the control of obesity. SCFAs have alleviating effects on inflammation and protective effects on gut barrier function. The mechanisms of the promotion of L cell proliferation and proglucagon mRNA expression in the gut may be involved in the effects of SCFAs [87,88]. Maslowski et al. [89] have also suggested that G-protein coupled receptor 43 (GPR43), an SCFA receptor, may mediate regulatory effects on inflammatory responses, thus providing protection from pro-inflammatory cytokines or D (LPS) [90,91]. An increasing amount of evidence has indicated that variations in the species composition of the gut microbiota are associated with human obesity [92,93], which may be due to increased expression of the fasting-induced adipose factor (Fiaf) gene in intestinal and adipose tissues [87].
Since it is generally impossible for dietary fiber to be absorbed into the plasma from the intestinal tract, these substances modulate the gut microbiota and improve host metabolism by acting as prebiotics. Moreover, dietary fiber from the shoot of the bamboo Dendrocalamus hamiltonii exhibits strong binding of water and oil as well as of cholesterol and bile acid [82]. Bitter melon also contains abundant phytochemicals, such as phenolic compounds and saponins. Polyphenols are endowed with significant antioxidant, antibacterial, and antifungal activities as well as the ability to inhibit enzymes involved in dietary carbohydrate and lipid digestion [94,95]. Polyphenols play an important role in modulating the gut microbiota [96–98]. In turn, the gut microbiota also metabolizes polyphenols. It is known that 90%–95% of dietary polyphenols cannot be absorbed from the intestinal tract, and that in order to be physiologically active, polyphenols are degraded to smaller phenolic compounds or monomers by microbial enzymes in the colon [99,100]. Several polyphenols participate in the inhibition of sodium-dependent glucose cotransporters (SGLT1) transporters and the intestinal glucose transporter 2 (GLUT2) [95,101]. Polyphenols have been reported to activate 50 adenosine monophosphate-activated protein kinase (AMPK) and downregulate adipogenic factors, including peroxisome proliferatoractivated receptor gamma (PPARγ) and CCAAT-enhancer-binding protein α (C/EBPα), thus suppress adipocyte differentiation in the 3T3-L1 cell line [102] (Table 1 and Fig. 3).
《Fig. 3》

Fig. 3. Changes and potential mechanisms of the major gut microbiota of obesity and T2DM when treated by TCMs. Blue font represents TCM, green font shows increased flora in the experiment, and red font shows decreased flora. The dotted arrow indicates the direction of action, and the text on the dotted line represents the underlying mechanism. Blue dotted lines indicate other potential mechanisms of action. GLP-2: glucagon-like peptide-2; HDAC: histone deacetylases; GPCRs: G protein-coupled receptors.
《3.4. TCMs, the gut microbiota, and type 2 diabetes》
3.4. TCMs, the gut microbiota, and type 2 diabetes
Type 2 diabetes (T2DM) is a common metabolic disease that is associated with low-grade inflammation and peripheral insulin resistance. Several TCMs have been shown to have anti-diabetic effects via different mechanisms, including, notably, by regulating the composition and function of the gut microbiota.
In the past several years, researchers—and particularly Chinese scholars—have attempted to apply different types of TCMs for the treatment of T2DM and to elucidate the mechanisms of these TCMs in terms of the gut microbiota. For example, according to previous studies, dietary components that are rich in polyphenols and plant polysaccharides (including cocoa beverages and green tea) can have anti-diabetic and anti-obesity effects [85], as can many other TCMs. Polysaccharide from Plantago asiatica L. (PLP) significantly reduced blood glucose, insulin, total cholesterol, triglycerides, non-esterified fatty acids, and malondialdehyde levels in T2DM rats, while significantly increasing HDL-cholesterol levels and antioxidant enzyme activity [103]. Stachyose (STS), which can be easily extracted from Stachys and legumes, can reduce serum lipopolysaccharide levels and IL-6 and TNF-α mRNA expression in a manner similar to metformin (MET) [104]. Treatment with resveratrol improves glucose homeostasis in obese mice [105]. Other ingredients from plants and TCMs, such as mushroom polysaccharides (distributed in medicinal mushrooms such as Ganoderma lucidum and Poria cocos, which can be both used as TCMs and consumed as functional foods) [106], Flos Lonicera (Lonicera japonica Thunb.) [107], and berberine [35], have also been shown to be effective at preventing obesity and insulin resistance in rats. In terms of clinical trials, few available reports involve TCMs, diabetes, and the microbiota all. One in-progress RCT involves intervention with Sancai Lianmei Particle [108] and another double-blinded, placebo-controlled RCT involves Gegen Qilian decoction (GQD), a Chinese herbal formula; both these compounds have been shown to be effective for the treatment of T2DM [109].
Although several previous studies have shown that various types of TCMs can lower serum glucose levels and have beneficial effects on T2DM in murine models, it has also been acknowledged that many TCMs exhibit poor absorption in bioavailability studies, and the mechanisms through which TCMs participate in the treatment of T2DM remain unknown. Therefore, for further investigation, some studies have focused on the gut microbiota as one of the multiple risk factors associated with this type of metabolic syndrome. The following mechanisms have been revealed in the abovementioned studies. Polyphenols can inhibit the activity of enzymes that digest carbohydrates and lipids in vitro [103]. Some polyphenols can inhibit the absorption of glucose by acting on transport proteins; these polyphenols have potential antibacterial activity and can inhibit the growth of pathogenic bacteria in the intestine. Meanwhile, the intestinal flora can metabolize polyphenols and regulate the biological activity of these compounds [85]. In obese mice, bitter melon powder (BMP) inhibits insulin resistance and exhibits anti-inflammatory effects by restoring the intestinal flora and intestinal metabolite levels [83]. PLP can significantly affect the diversity of the colon microflora in rats and alter the abundance of bacteria such as Bacteroides (e.g., Bacteroides vulgates), Lactobacillus fermentum, and Prevotella pneumophila. The anti-diabetic effects of PLP may be associated with altered intestinal flora and increased levels of SCFAs [103]. STS, an ingredient that is extracted from Rehmanniae Radix, has similar effects to those of MET, causing alterations in the intestinal flora and selective enrichment of key species. At the genus level, STS selectively increases enrichment of the genus Phascolarctobacterium and four additional species, while MET mainly enriches four species, including the genus Sutterella. STS also reduces the expression of IL-6 and TNF-α mRNA in the pancreas by affecting the key species of intestinal flora, which is similar to the mechanism associated with MET [104]. In addition, resveratrol intake can change the composition and function of the intestinal microflora in mice. It is notable that the abundance of bacteria such as Lachnospiraceae and Akkermansia decreases, and the abundance of Bacteroidetes increases. After fecal transplantation into obese mice using resveratrol-treated rats as donors, the glucose homeostasis of the mice was improved [105]. Active substances in plants and mushrooms, such as celastrol, a compound extracted from the roots of the thunder god vine, can reduce appetite by reducing leptin resistance; they can also regulate lipid absorption and metabolism, increase insulin sensitivity, increase heat production, and change the intestinal flora [106]. The efficacy of Lianmei granules and acarbose, has yet to be evaluated in clinical trials of the composition of patients’ gut microbiota. [108]. However, another clinical RCT showed that GQD changes the intestinal microbiota when the symptoms of T2DM are treated; changes in the gut microbiota occurred prior to the improvement of diabetic symptoms, and the abundance of beneficial bacteria in the intestine increased [109]. All types of TCMs lead to changes in the gut microbiota to varying degrees, either in vitro or in vivo (Table 1 and Fig. 3).
《4. Perspectives》
4. Perspectives
As the above results show, various types of TCM have numerous functions in preventing and treating different diseases associated with changes in the gut microbiota. Although research on the interactions between TCMs and the gut microbiota is still in the preliminary stage, previous results have laid a scientific foundation for TCM research. After exposure to TCMs, the intestinal microbiota undergoes structural changes, and the intestinal epithelial cells of the host respond to these changes by sending signals to the enteric nervous system and then to the brain. Simultaneously, the enteric nervous system receives information from the brain to modulate microbial functions [18,110]. The enrichment of beneficial gut microbes and the decrease in the abundance of harmful gut microbes occur before the improvement of disease symptoms, which indicates that recovery of the balance of the gut microbiota may promote the improvement of disease symptoms, rather than being a mere result of symptom improvement [109]. The biotransformation of the gut microbiota by TCM components, the multicomponent interactions among TCMs mediated by the gut microbiota and the composition of the host immune system, and the promotion and inhibition of the gut bacteria by TCMs together indicate that the gut microbiota is a potential therapeutic target for TCMs. Previously, absorption was considered necessary for TCM function; now, however, it has been clearly shown that TCM components (e.g., polysaccharides) can affect host physiological and pathological conditions via the gut microbiota, even without absorption. Therefore, the interactions of TCMs with the gut microbiota should be taken into account in future drug discovery research [1].
In future, further studies are required with more advanced experimental design, such as direct microbial analysis of the TCM-targeted gut microbiota, and the development of a precise research model of the gut microbiota. The results of such research will further reveal the interactions between TCMs and the gut microbiota, thus providing novel insight and guiding TCM-based drug discovery [1]. In addition, the superorganism view of the human body, which includes the gut microbiota, provides a completely new systems concept for managing human health holistically.
However, certain problems remain associated with current TCM research: First, we lack a sufficient understanding of the actual modes of TCM action. Second, occasional manifestations of toxicity and a lack of well-controlled clinical trials have prevented TCMs from becoming mainstream medicines. The ‘‘personalized and holistic nature” of TCMs has also contributed to difficulties in performing the randomized, placebo-controlled, and double-blinded trials used in mainstream medicine [111]. All of these factors have hindered the use of TCMs in modern medical applications; therefore, additional modern tools and methods are needed in further TCM research.
《Acknowledgements》
Acknowledgements
We thank the Key Program of the National Natural Science Foundation of China (81330011), the National Natural Science Foundation of China (81790630, 81790631, and 81790633), the Zhejiang Provincial Natural Science Foundation of China (R16H260001), and the National Basic Research Program of China (2013CB531401).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Yan-Meng Lu, Jiao-Jiao Xie, Cong-Gao Peng, Bao-Hong Wang, Kai-Cen Wang, and Lan-Juan Li have no conflicts of interest to declare, and the manuscript has been approved for publication by all authors.














 京公网安备 11010502051620号
京公网安备 11010502051620号




