《1. Introduction》
1. Introduction
Tetragonal ferromagnetic compounds of the ThMn12 type—that is, RT12 compounds in which R stands for rare earth element and T is mostly iron (Fe)—were recognized as promising hard magnetic materials as early as the 1980s. At that time, however, another iron-based 4f–3d compound, Nd2Fe14B, was being rapidly developed into powerful permanent magnets. Its higher saturation magnetization, relative ease of developing a useful coercivity (Hc), and phase equilibria favoring liquid-phase sintering and hot plastic deformation ensured the primary role of Nd–Fe–B, which now dominates the market of high-power permanent magnets. Recently, the rapidly growing demand for magnets operating at 150–200 °C and the 2010–2011 rare-earth supply crisis instigated an investigation of “new materials” [1], including the not-so-new RT12. Since the composition of these materials has the lowest rare-earth content among the 4f–3d compounds, magnets based on these compounds may be less reliant on critical raw materials. Unlike Nd2Fe14B, RFe12-type compounds are characterized by a negative value of the crystal field parameter 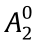 ; thus, they exhibit the strongest uniaxial magnetocrystalline anisotropy for the rare earths R with a positive Stevens coefficient
; thus, they exhibit the strongest uniaxial magnetocrystalline anisotropy for the rare earths R with a positive Stevens coefficient  , such as samarium (Sm). At present, Sm is believed to be less vulnerable to supply restrictions than any other rare-earth metal [2]. If binary RFe12 alloys were realized, they would have the highest Fe content among the 4f–3d compounds and would thus be expected to exhibit the largest values of magnetization and energy density. One feature distinguishing current approaches to the development of RT12 hard magnetic materials from past approaches is that the former are supported by density functional theory (DFT) calculations and, most recently, by molecular dynamics simulations regarding the selection of stable structures with high magnetization. Many recent and ongoing experiments are pursuing RT12 compounds with a higher concentration of magnetic Fe and cobalt (Co) atoms. Yet another avenue of research is aimed at the incorporation of more abundant and underused rare earths cerium (Ce) and lanthanum (La), or non-critical zirconium (Zr), even though the nonmagnetic atoms of these elements would not contribute to the magnetization and anisotropy of the compounds. The major challenge, however, lies in converting RFe12 compounds with promising fundamental magnetic properties into functional and preferably fully dense and anisotropic magnets, which could compete with the nearly perfected Nd–Fe–B. In this paper, we provide an overview of our ongoing research in the context of other recent developments in RT12 compounds and magnets.
, such as samarium (Sm). At present, Sm is believed to be less vulnerable to supply restrictions than any other rare-earth metal [2]. If binary RFe12 alloys were realized, they would have the highest Fe content among the 4f–3d compounds and would thus be expected to exhibit the largest values of magnetization and energy density. One feature distinguishing current approaches to the development of RT12 hard magnetic materials from past approaches is that the former are supported by density functional theory (DFT) calculations and, most recently, by molecular dynamics simulations regarding the selection of stable structures with high magnetization. Many recent and ongoing experiments are pursuing RT12 compounds with a higher concentration of magnetic Fe and cobalt (Co) atoms. Yet another avenue of research is aimed at the incorporation of more abundant and underused rare earths cerium (Ce) and lanthanum (La), or non-critical zirconium (Zr), even though the nonmagnetic atoms of these elements would not contribute to the magnetization and anisotropy of the compounds. The major challenge, however, lies in converting RFe12 compounds with promising fundamental magnetic properties into functional and preferably fully dense and anisotropic magnets, which could compete with the nearly perfected Nd–Fe–B. In this paper, we provide an overview of our ongoing research in the context of other recent developments in RT12 compounds and magnets.
《2. Crystal structure, magnetism, and early efforts to develop Hc》
2. Crystal structure, magnetism, and early efforts to develop Hc
Fig. 1 depicts the ThMn12 unit cell; the cell incorporates two formula units. The two large thorium (Th) atoms occupy a single  site and the 24 small manganese (Mn) atoms are evenly distributed between the
site and the 24 small manganese (Mn) atoms are evenly distributed between the  sites. The tetragonal RT12 structure—like the rhombohedral and hexagonal R2T17 structures, the monoclinic R3T29 structure, and the disordered hexagonal solid solution known for its TbCu7 prototype—can be derived from the hexagonal RT5 structure by replacing part of the R atoms with pairs of the T atoms. When T = Fe, a third element, M, is needed to stabilize the RT12 structure, as R(Fe,M)12. The large transition metal atoms (M = Ti, V, Nb, Mo, Ta, W) occupy the 8i sites of R(Fe,M)12; these sites are characterized by the largest Wigner–Seitz cell. The sp atoms (M = Al, Si) prefer the 8f sites, which allow for two direct bonds with the R atoms [3,4]. Up to one light element atom (i.e., H, N, or C) may occupy the interstitial position in the RT12 cell, but the resulting modified lattice is metastable; it can only exist as long as the formation of the more stable rare-earth hydrides, nitrides, or carbides is suppressed by kinetic factors. Interstitial modification with nitrogen, in particular, increases the Curie temperature and changes the sign of the
sites. The tetragonal RT12 structure—like the rhombohedral and hexagonal R2T17 structures, the monoclinic R3T29 structure, and the disordered hexagonal solid solution known for its TbCu7 prototype—can be derived from the hexagonal RT5 structure by replacing part of the R atoms with pairs of the T atoms. When T = Fe, a third element, M, is needed to stabilize the RT12 structure, as R(Fe,M)12. The large transition metal atoms (M = Ti, V, Nb, Mo, Ta, W) occupy the 8i sites of R(Fe,M)12; these sites are characterized by the largest Wigner–Seitz cell. The sp atoms (M = Al, Si) prefer the 8f sites, which allow for two direct bonds with the R atoms [3,4]. Up to one light element atom (i.e., H, N, or C) may occupy the interstitial position in the RT12 cell, but the resulting modified lattice is metastable; it can only exist as long as the formation of the more stable rare-earth hydrides, nitrides, or carbides is suppressed by kinetic factors. Interstitial modification with nitrogen, in particular, increases the Curie temperature and changes the sign of the 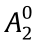 crystal field parameter from negative to positive. Thus, unlike R(Fe,M)12 compounds, R(Fe,M)12Nx nitrides exhibit a strong uniaxial anisotropy when R = praseodymium (Pr) or neodymium (Nd) (
crystal field parameter from negative to positive. Thus, unlike R(Fe,M)12 compounds, R(Fe,M)12Nx nitrides exhibit a strong uniaxial anisotropy when R = praseodymium (Pr) or neodymium (Nd) ( < 0) [3].
< 0) [3].
《Fig. 1》
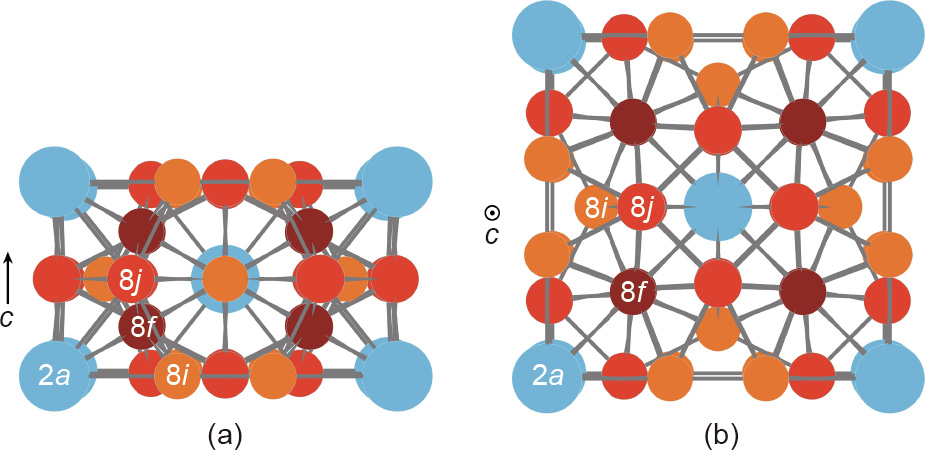
Fig. 1. Schematic representation of a ThMn12 unit cell along (a) the [100] direction and (b) the [001] direction c.
Early attempts to develop plausible hard magnetic properties in the 1:12 alloys produced an Hc of 11–12 kOe (1 Oe = 79.6 A·m-1 ) in isotropic melt-spun or high-energy-milled Sm(Fe,Ti,V)12 alloys [5,6] and a full-density-projected maximum energy product (BH )max of 21 MG·Oe (1 MG·Oe = 7.96 kJ·m-3 ) in Nd(Fe,Mo)12Nx anisotropic powders [7]. Experiments with sintering powdered Sm(Fe,M)12 alloys into fully dense magnets were not very successful, even when the sintering was performed in a Sm atmosphere [8].
《3. RFe12 materials since 2010》
3. RFe12 materials since 2010
《3.1. Computational approaches》
3.1. Computational approaches
First-principle calculations based on DFT make it possible to search for promising R(Fe,M)12X compounds over a great variety of R, M, and X elements [9,10]. However, the first attempt at true high-throughput computational screening, in which the structures were generated and analyzed automatically [10], was undermined by inaccurate assumptions about the site occupancies of the M atoms (only the 8i sites) and the valence state of the Ce ions (Ce3+ instead of the mixed-valence state between Ce3+ and Ce4+), and by incorrect evaluation of the stability of the compound based on the formation energy of only this compound. A specific target of these theoretical studies was the interstitial modification, particularly with X elements such as oxygen (O), fluorine (F) [11], and lithium (Li) [12], which would be difficult to study experimentally. A strong uniaxial anisotropy that is less temperature dependent than that of the NdFe12N nitride was predicted for the hypothetical SmFe12Li [12]. It is unlikely, however, that these interstitially modified compounds—even if synthesized—will be more stable than the R(Fe,M)12H hydrides and R(Fe,M)12N nitrides; thus, the range of their applications will be rather limited. An arguably more worthwhile aim is to identify the conditions for stable R(Fe,M)12 compounds with minimal or no substitution for the magnetic Fe atoms. The formation of R(Fe,M)12 structures with various M elements, the stabilities of RFe12 with various R elements including scandium (Sc), Zr, and hafnium (Hf), and the effect of hydrostatic pressure have been analyzed [13,14]. These calculations have confirmed what was already known from experiments: For any R, the hypothetical binary RFe12 structure is less stable than the R2Fe17 structure. At the same time, the difference between the RFe12 and R2Fe17 formation energies is reduced for R = Nd or Sm if these elements are partially replaced with Zr, yttrium (Y), dysprosium (Dy), holmium (Ho), erbium (Er), or thulium (Tm). Thus, although substitution for the R element does not make RT12 structures stable, it may decrease the minimum necessary substitution for the T element. In addition to DFT calculations, molecular dynamics, which uses the classical equations of motion to predict the dynamic evolution of atoms in a crystal structure, has recently become another approach to assess the stability of the R(Fe,M)12 structure with a minimal percentage of M [15].
《3.2. Epitaxially grown RFe12 and R(Fe,Co)12 films with high magnetization》
3.2. Epitaxially grown RFe12 and R(Fe,Co)12 films with high magnetization
The presence of a binary SmFe12 compound stabilized with an underlayer in an epitaxially grown thin film was first reported in 1991 [16]. As expected for an undiluted Fe component, it exhibited a high but not exceptional saturation magnetization. The full significance of excluding the third structure-stabilizing element became clear only in the last three years, after the preparation and characterization of NdFe12Nx and Sm(Fe,Co)12 epitaxial films [17–19]. Table 1 compares the basic magnetic properties, Curie temperature (TC), saturation magnetization (4πMs), and magnetic anisotropy field (Ha) of these films with the properties of Nd2Fe14B and Nd2(Fe,Co)14B. The comparison demonstrates that ThMn12- type compounds exhibit potential for greater magnetic energy density. The theoretical limit of the (BH )max of SmFe9.6Co2.4 films can be estimated as (2πMs) 2 = 79 MG·Oe, which is a record high value. At the practically important elevated temperatures of 150– 200 °C, this superiority becomes even greater, due to the low TC of Nd2Fe14B and the highly detrimental effect of Co on the Ha(T ) dependence of Nd2(Fe,Co)14B [20].
《Table 1》
Table 1 Room-temperature magnetic properties and Curie temperatures reported for selected Nd2Fe14B-type and ThMn12-type compounds.

a Some of the reports indicate the presence of impurity phases, but do not account for their effect on the composition of the principal phase.
b Form in which the material can be synthesized; “any” indicates an absence of known restrictions.
High-magnetization thin films exhibit an Hc of 4–5 kOe [18,29]. Infiltration of the films with copper (Cu) and gallium (Ga), aimed at the magnetic insulation of Sm(Fe,Co)12 crystallites, reportedly increases the Hc to 8 kOe, even though no continuous grainboundary phase has actually been formed [29]. These Hc values are still quite low compared with the values of more than 30 kOe that are developed in the Nd–Fe–B thin films [30]. The real problem, however, is not the relatively low Hc, but the current inability to prepare bulk NdFe12Nx and Sm(Fe,Co)12 alloys without the stabilizing effect of an underlayer. As long as these compounds are only available as thin films, they cannot be used as permanent magnets: The shape of the thin films does not allow for the utilization of their stored magnetic energy.
《3.3. SmFe12–xVx compounds with increased magnetization》
3.3. SmFe12–xVx compounds with increased magnetization
Early studies of RFe12–xMx compounds established the minimum x values as approximately 0.5 for M = molybdenum (Mo) or tantalum (Ta), 0.7 for M = niobium (Nb) or tungsten (W), 1 for M = titanium (Ti), 1.5 for M = vanadium (V) or chromium (Cr), and 2 for M = silicon (Si). For reasons that are still not well understood, Sm(Fe,V)12 alloys are most susceptible to the development of viable coercivities. In an attempt to obtain SmFe12–xVx alloys with an x smaller than reported earlier [3,31,32], we have studied the phases and magnetic properties in alloys with x varying from 0.5 to 2. The alloys were prepared by arc-melting followed by homogenization at temperatures in the range of 900–1100 °C for 1–4 d. After the appropriate homogenization, a nearly pure ThMn12-type structure was obtained for x = 1; the corresponding X-ray diffraction (XRD) spectrum is shown in Fig. 2(a). The strong [002] peak observed in the spectrum recorded for the oriented powder in Fig. 2(b) confirms that the magnetocrystalline anisotropy of the compound is uniaxial. The reduction of x in the SmFe12–xVx compound from 2 to 1 increases the TC from 321 to 361 °C, the saturation magnetization from 83 emu·g-1 (1 emu·g-11 =1A·m2 ·kg-11) (8.1 kG) to 115 emu·g-1 (11.2 kG), and the anisotropy field from 98 to 110 kOe.
《Fig. 2》
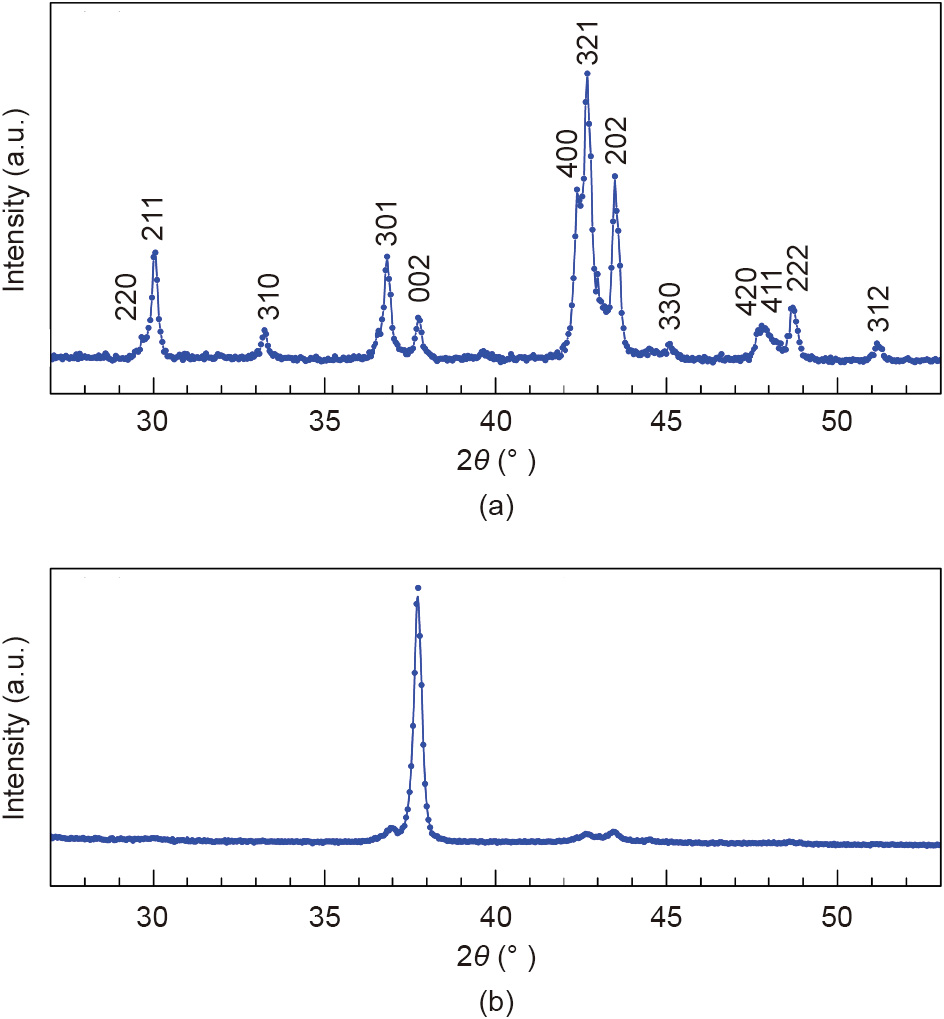
Fig. 2. XRD spectra of SmFe11V (a) randomly oriented and (b) field-oriented powders. θ : the X-rays incident angle.
《3.4. Nitrogenization of R(Fe,M)12 compounds with M = V, Mo, and Si》
3.4. Nitrogenization of R(Fe,M)12 compounds with M = V, Mo, and Si
Despite the vast amount of data that has been gathered on Cosubstituted R(Fe1–xCox,M)12 alloys and, on the other hand, on interstitially modified R(Fe,M)12Ny nitrides, the effect of Co substitution on the properties of these nitrides remained the subject of controversy for a long time. A recent study [33] demonstrated that small Co substitutions improve all the basic magnetic properties of the NdFe10.5–xCoxMo1.5Ny nitrides, whereas large Co substitutions are detrimental not only for the 4πMs and Ha, but even for the TC. The improvement was confirmed for the NdFe10.5–xCoxV1.5Ny nitrides, with the substitution x = 0.9 increasing the saturation magnetization by 11%, to 138.5 emu·g-1 [33].
Until recently, interstitial modification with nitrogen had only been reported in R(Fe,M)12 compounds with M represented by the transition metals. Apparently, the gas–solid nitrogenization reaction does not occur at 1 atm (1 atm = 1.01 × 105 Pa) nitrogen pressure when M = Si, Al. However, after increasing the pressure to 20 atm, CeFe10Si2Ny [34] and Nd0.6Zr0.4Fe10Si2Ny nitrides were successfully synthesized. The latter nitride was found to have an Ha of 53 kOe compared with 29 kOe for the parent compound. Fig. 3 presents thermogravimetric scans demonstrating a twophase formation of the interstitially modified structure that is characterized by a higher TC.
《Fig. 3》
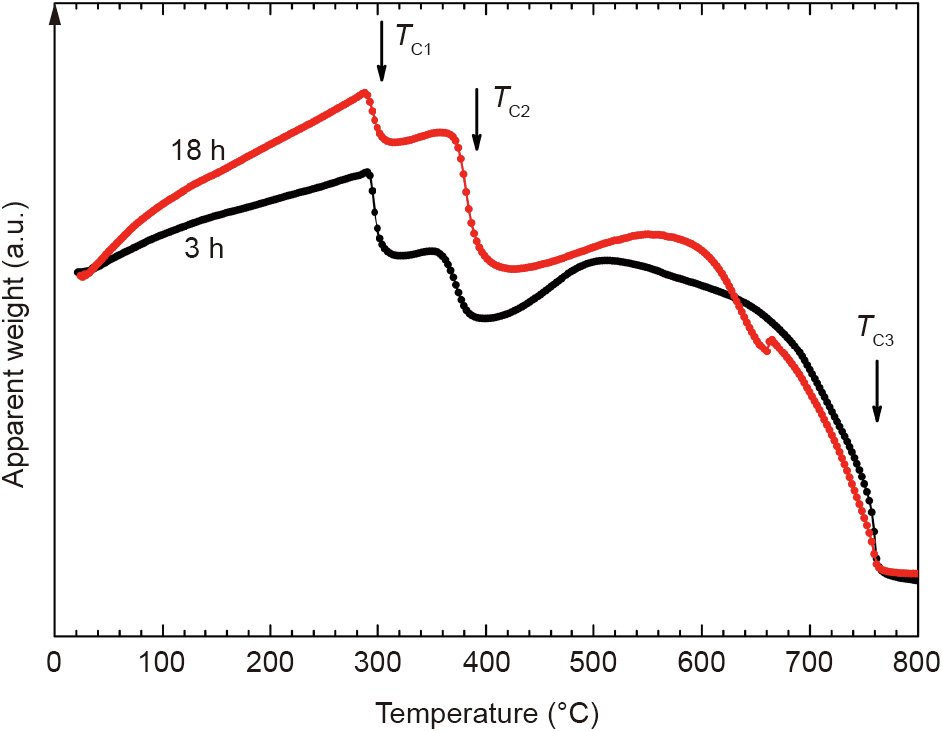
Fig. 3. Apparent weight of Nd0.6Zr0.4Fe10Si2Ny alloys heated in a thermogravimetric analyzer in a constant magnetic field after nitrogenization at 550 °C for 3 h (black) and 18 h (red) under 20 atm nitrogen gas. TC1, TC2, and TC3 indicate the Curie temperatures of the parent phase, nitride phase, and body-centered cubic (bcc) Fe–Si impurity phase, respectively.
《3.5. R1–vAv(Fe,Co,M)12 compounds with increased magnetization and reduced R content (A = Zr, Y)》
3.5. R1–vAv(Fe,Co,M)12 compounds with increased magnetization and reduced R content (A = Zr, Y)
Recent experimental findings have indicated that the ThMn12- type structure R(Fe,Co)12–xTix, R = Nd or Sm can be obtained with x < 1 when part of R is replaced by Zr [23,24,28] or Y [26]. This effect was explained through the similarity between the local lattice changes for Zr atoms occupying the  sites and Ti atoms occupying the
sites and Ti atoms occupying the  sites [35]. DFT calculations [13] have also indicated that partial replacement of Nd or Sm with Zr and Y should decrease the difference between the higher formation energy of the RFe12 structure and the lower formation energy of the R2Fe17 structure. Table 1 presents the properties reported for Zr- and Y-substituted R1–vAv(Fe,Co)12–xTix compounds with x < 1. The room-temperature saturation magnetization of these compounds approaches or even exceeds that of Nd2Fe14B; at 150–200 °C, both the 4πMs and Ha of ThMn12-type compounds are superior [27]. It should be noted that the alloys prepared with x = 0.5 contained impurity phases including the α-Fe phase; it was claimed that the 4πMs values listed in Table 1 accounted for the impurities, but the actual compositions of the corresponding ThMn12-type phases are likely to have x > 0.5. No experiments aimed at the development of an Hc in these “Ti-lean” compounds have been reported yet, and the presence of the soft magnetic α-Fe phase in alloys with x = 0.5 is unfavorable for such efforts. Our attempt to prepare magnetically hard “Ti-lean” alloy via mechanochemistry is described in Section 4.3.
sites [35]. DFT calculations [13] have also indicated that partial replacement of Nd or Sm with Zr and Y should decrease the difference between the higher formation energy of the RFe12 structure and the lower formation energy of the R2Fe17 structure. Table 1 presents the properties reported for Zr- and Y-substituted R1–vAv(Fe,Co)12–xTix compounds with x < 1. The room-temperature saturation magnetization of these compounds approaches or even exceeds that of Nd2Fe14B; at 150–200 °C, both the 4πMs and Ha of ThMn12-type compounds are superior [27]. It should be noted that the alloys prepared with x = 0.5 contained impurity phases including the α-Fe phase; it was claimed that the 4πMs values listed in Table 1 accounted for the impurities, but the actual compositions of the corresponding ThMn12-type phases are likely to have x > 0.5. No experiments aimed at the development of an Hc in these “Ti-lean” compounds have been reported yet, and the presence of the soft magnetic α-Fe phase in alloys with x = 0.5 is unfavorable for such efforts. Our attempt to prepare magnetically hard “Ti-lean” alloy via mechanochemistry is described in Section 4.3.
In Zr-substituted R1–vZrvFe10–xSix compounds, the x value cannot be decreased below 1.6, which is much higher than in the compounds stabilized by Ti. What is remarkable about the Si-stabilized ThMn12-type structures is that they can sustain a complete replacement of the rare-earth element R with Zr [36]. The rareearth-free Zr(Fe,Si)12 compounds are, apparently, metastable and their anisotropy is believed to be too weak for permanent magnet materials. However, as an answer to the overreliance of permanent magnets on the ‘‘critical” rare-earth elements, very rare-earth-lean R1–xZrx(Fe,Si)12 compounds with R = Nd [37] and Sm [38] may be of interest.
《4. Development of RFe12 magnets》
4. Development of RFe12 magnets
《4.1. Sintering》
4.1. Sintering
With the exception of “intrinsically hard” R(Co,M)5 compounds, the development of Hc in uniaxially anisotropic ferromagnetic 4f– 3d compounds is achieved through refinement of the crystallites. In the practice of permanent magnet manufacturing, this refinement typically involves rapid solidification or powder metallurgy. Although the former has been proven to work for ThMn12-type alloys [5], only the latter—that is, milling followed by magneticfield alignment and sintering—can reliably ensure textured and fully dense materials. Traditional sintering of Nd(Fe,M)12Nx nitrides is ruled out by the inherent instability of these compounds. Apart from the high vapor pressure of Sm [8], the most obvious obstacle to manufacturing sintered magnets with Sm(Fe,M)12 alloy as the principal phase is the soft magnetic phases that surround that compound on the equilibrium phase diagram [3]. In contrast, sintering of Nd-Fe-B magnets is greatly facilitated by the eutectic reaction between the Nd2Fe14B and Nd phases; the emerging liquid assists in the densification of the press– compacts and allows for a partial or even complete insulation of the Nd2Fe14B grains with non-magnetic phases.
La is unique among the rare-earth elements in that it does not form binary compounds with Fe, and has been shown to avoid ThMn12-type structures [38]. It is, therefore, conceivable that the addition of La to R(Fe,M)12 alloys will lead to a eutectic mixture of the R(Fe,M)12 and La phases, thus facilitating both the densification and development of Hc through sintering. To test this hypothesis, a Sm8La5Fe80Ti7 alloy, which may also be presented as (Sm0.084Fe0.842Ti0.074)95La5, was prepared via arc-melting and homogenized for 3 h at 1050 °C. At this stage, according to the XRD characterization shown in Fig. 4(a) (spectrum ①), the ThMn12-type phase co-exists with what appears to be metallic La. The alloy was ball-milled under toluene for 1.5–10 h. The resulting fine powders were wet-pressed in a magnetic field, then sealed in argon-filled quartz capsules and sintered for 3 h at 1050 °C. The phases and properties of the sintered alloys appear to be strongly influenced by the oxidation of the highly reactive metallic La during the milling. As the milling time increased, an increasing amount of a rare-earth oxide (perhaps La oxide?) could be detected in the sintered alloys, while the XRD reflections corresponding to the double hexagonal close packed (dhcp) La decreased and eventually disappeared (Fig. 4(a)). It is believed that the eutectic reaction between the R(Fe,Ti)12 and La phases facilitated densification of the powders milled for 1.5–3 h; the corresponding density values are presented in Fig. 4(b). The electron micrographs shown in Fig. 5 reveal that the 3–20 μm as-milled particles with sharp edges evolve into 5–30 μm grains of the R(Fe,Ti)12 phase with well-developed facets. The minority R-rich phases are characterized by very large La/R ratios—0.92 for the oxide phase and 0.98 for the phase found to form continuous layers between the grains of the main phase. At a La/R ratio of about 0.22, the main phase is depleted with La compared with the alloy composition; microscopy also reveals small—undetectable with XRD—amounts of the α-(Fe,Ti) and TiO2 phases. Both the powder particles and grains of the sintered alloy were rather coarse after milling for 1.5 h, so the latter exhibited a very low Hc of 0.1 kOe. After milling for 10 h, nearly all of the La phase of the starting alloy was oxidized, and the sintering did not lead to any significant densification (Fig. 4). However, the 10 h of milling resulted in uniform particles approximately 1 μm in size, leading to fine, 3–5 μm grains after the sintering (the micrographs are not shown). This refinement may explain the higher Hc of 0.42 kOe. This Hc, however, is still too low—either because the low density leaves too many grain surfaces exposed or because significant amounts of the magnetically soft α-(Fe,Ti) phase and TiO2 precipitates facilitate reversal of the magnetization. The increased amounts of the α-(Fe,Ti) and TiO2 phases indicate that the longer milling time led to the oxidation not only of the La phase, but also of a part of the rare-earth elements in the R(Fe,Ti)12 phase.
《Fig. 4》
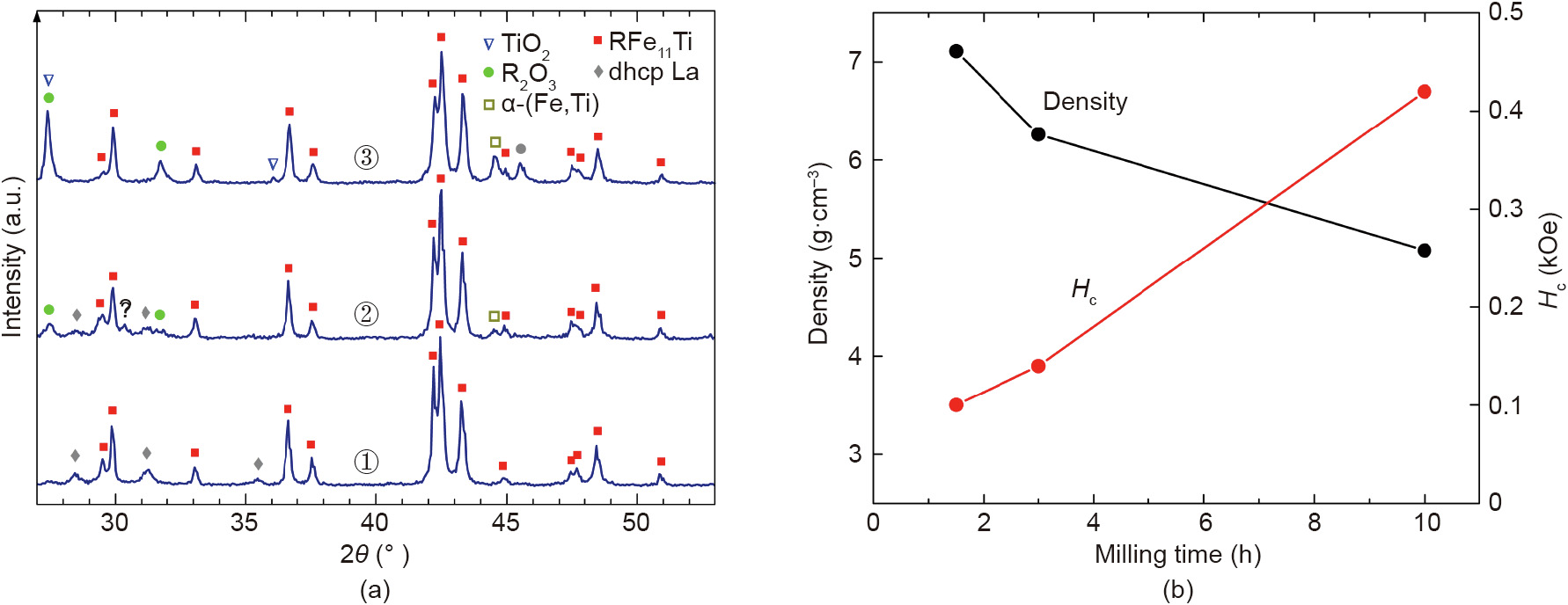
Fig. 4. Characterization of (Sm0.084Fe0.842Ti0.074)95La5 alloy subjected to homogenization, ball-milling, and sintering. (a) XRD spectra of ① homogenized alloy, ② alloy sintered after milling for 1.5 h, and ③ alloy sintered after milling for 10 h; (b) properties of the sintered alloys as a function of milling time. Double hexagonal close packed (dhcp) structure of metallic La was identified tentatively.
《Fig. 5》

Fig. 5. Micrographs of (Sm0.084Fe0.842Ti0.074)95La5 alloy after (a) milling for 1.5 h (secondary electrons image) and (b) subsequent sintering (backscattered electrons image). Black areas in (b) correspond to voids.
Thus, even though we were apparently able to induce a lowmelting-temperature eutectic, and this eutectic allowed for a more complete sintering, the high reactivity of La conflicted with the development of Hc through the refinement of the crystallites in this experiment.
《4.2. “Bulk” magnetic hardening》
4.2. “Bulk” magnetic hardening
Specific alloy systems such as Sm–Co–Fe–Cu–Zr or Pr–Fe–B–Cu allow for the manufacturing of functional magnets via a simple and less costly alternative to sintering and rapid solidification—via the recrystallization of regularly cast ingots. If formation of the hard magnetic phase is initially suppressed, subsequent heat treatment can be tuned to ensure that the phase crystallizes with the proper morphology. The only known R(Fe,M)12 alloy susceptible to bulk hardening is Sm(Fe,V)12; it solidifies into a mixture of the α-(Fe, V) and SmFe2 phases and, after controlled crystallization of the SmFe10V2 phase, develops an Hc up to 3.7 kOe [39]. We were able to achieve bulk hardening in alloys prepared from Fe, Ti, and an inexpensive mixture of 65% Ce and 35% La metals (the mixture is close to the naturally occurring ratio of these underused rare earth elements) utilizing the tendency of La to form low-meltingtemperature eutectics, as described in Section 4.1. Fig. 6 shows microstructures of the alloy designed as a hypothetical mixture of the CeFe11Ti and La phases. The as-prepared ingot consisted mostly of bcc α-(Fe,Ti) and RFe2 phases (the XRD data are not shown). The dhcp La (or La–Ce) phase was also observed, along with a small amount of the R(Fe,Ti)12 phase. Differential thermal analysis (DTA) of the as-prepared alloy (Fig. 7, inset) revealed a probable eutectic reaction involving the La–Ce phase at 718 °C (in the binary alloy systems, the eutectic reaction between La and Fe occurs at 780 °C, and that between Ce and CeFe2 occurs at 592 °C).
《Fig. 6》
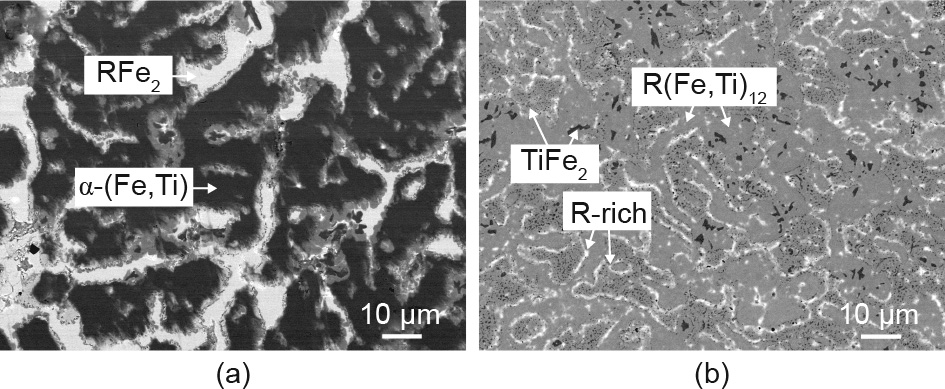
Fig. 6. Backscattered electrons micrographs of (Ce0.09Fe0.83Ti0.08)95.5La4.5 alloy. (a) As-cast; (b) annealed at 725 °C for 48 h.
《Fig. 7》
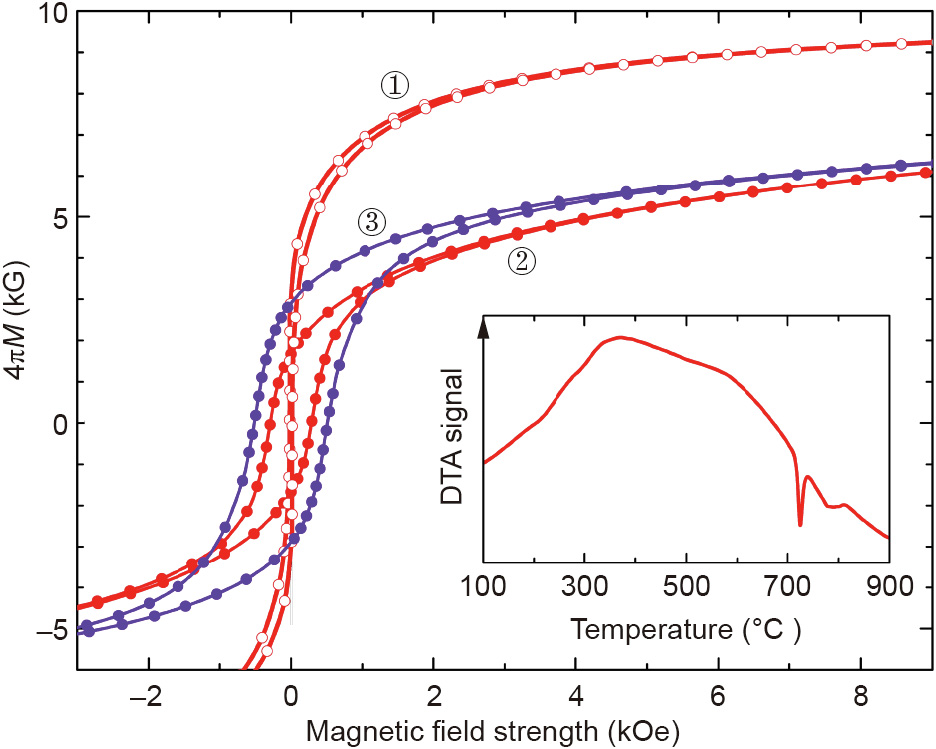
Fig. 7. Room-temperature magnetic hysteresis loops of ① as-cast (Ce0.09Fe0.83Ti0.08)95.5La4.5 alloy; ② the same alloy annealed at 725 °C for 48 h; ③ (Ce0.07Sm0.02Fe0.83Ti0.08)95.5La4.5 alloy annealed at 725 °C for 0.5 h. Inset: DTA scan of alloy ①.
Heat-treated Ce–La–Fe–Ti alloys develop a microstructure consisting of CeFe11Ti grains surrounded by La-rich phases (Fig. 6(b)). The fine black precipitates were identified as the non-magnetic TiFe2 phase. This microstructure results in obvious magnetic hardening, although because the Ha of the CeFe11Ti phase is only 17 kOe [40] and some of the grains are larger than 10 μm, the Hc is only 0.3 kOe (Fig. 7). A partial replacement of Ce with Sm can increase the Hc to at least 0.5 kOe. Only a small part of the Ce, however, can be replaced; otherwise, crystallization of the R(Fe,Ti)12 phase is no longer suppressed in the as-prepared alloys. We also reproduced the bulk hardening in the Sm–Fe–V alloys [39] and attempted to improve the microstructure developing in these alloys by adding La. Although a uniform microstructure of fine, 1–10 μm grains surrounded by a La-rich phase was obtained (Fig. 8, inset), the maximum Hc of 3.3 kOe was not higher than in the earlier report [39]. It may be noted that annealing at different temperatures allowed us to suppress and stabilize the RFe2 minority phase; the Hc was found to be consistently higher when the RFe2 phase was present in the alloy.
《Fig. 8》

Fig. 8. Room-temperature demagnetization curves of (Sm0.12Fe0.75V0.13)98La2 alloy annealed at 700 °C for 20 h. Inset: backscattered electrons micrograph showing Sm(Fe,V)12 grains separated by a La-rich phase.
《4.3. Mechanochemistry》
4.3. Mechanochemistry
Instead of refining the grains or particles of a pre-manufactured alloy, it is possible to obtain ultrafine single crystals directly from elemental components by reducing a mixture of the corresponding oxides with metallic calcium (Ca). Since the calciothermic reduction requires temperatures in excess of 800 °C, special measures must be taken to prevent growth and sintering of the newly synthesized particles. In 1997, 3–8 μm particles of Nd(Fe,Mo)12 alloy were obtained by reducing chemically synthesized nanoscale oxide precursors [41]. More recently, a mechanochemical approach, which achieves the initial refinement through mechanical activation, was employed to prepare submicron (Sm,Ce)(Fe,Co)11Ti [42] and (Sm,Ce,Zr)Fe10Si2 [43] particles. The Sm0.7Zr0.3Fe10Si2 particles exhibited a remarkable (for the ThMn12-type alloys) combination of susceptibility to magnetic-field alignment and a high Hc of 10.8 kOe; when projected onto a full-density magnet, their (BH )max was 13.8 MG·Oe.
We used mechanochemistry in our attempt to develop an Hc in the "i-lean” high-magnetization Sm1–yZry(Fe,Co)12–xTix alloys, which were reviewed in Section 3.5. A blend of Sm2O3, ZrO2, Fe2O3, TiO2, and Co powders was subjected to high-energy ballmilling, together with Ca granules and a CaO powder that was added as a dispersant. The mechanically activated mixture was then annealed for 5 min at 1100–1150 °C to complete the reduction. The alloy particles were collected after washing off the dispersant and byproducts of the synthesis [43]. Fig. 9 presents the magnetization curves recorded for the oriented particles. The Hc of 2–3 kOe was lower than that of the similarly prepared SmFe11Ti particles [42], and the less-than-perfect alignment did not allow for the remanent magnetization that could be expected based on the properties reported for the alloy (Table 1). It is likely that further tuning of the process will more completely realize the high potential of the alloys, although it must be noted that no working procedure has been developed yet to convert the mechanochemically synthesized powders into fully dense permanent magnets.
《Fig. 9》

Fig. 9. Room-temperature demagnetization curves of field-oriented Sm0.9Zr0.1(Fe0.75Co0.25)11.35Ti0.65 particles (nominal composition) measured parallel (filled symbols) and perpendicular (open symbols) to the alignment direction. The particles were prepared via mechanical activation of an oxide-calcium mixture followed by annealing at 1100 °C (blue squares) or 1150 °C (red circles) and washing. Inset: particles synthesized at 1100 °C.
《5. Concluding remarks》
5. Concluding remarks
This review highlights the wide gap that exists between the excellent intrinsic properties reported for several Fe(Co)-rich ThMn12-type compounds and the marginal hard magnetic properties of the corresponding alloys. It may never be possible to synthesize in bulk the RFe12 and R(Fe,Co)12 compounds that can be stabilized in epitaxially grown thin films. Furthermore, the x = 0.5 value for Ti reported in R1–vZrv(Fe,Co)12–xTix compounds may be too small to allow the development of functional magnets; a somewhat larger x seems to be needed in order to avoid the presence of a magnetically soft α-Fe phase (the micromagnetics simulations in Ref. [44], which concluded that a few percent of the α-Fe phase can be tolerated, was performed by assuming finer grains than what the real magnets are likely to have). However, even easy-tosynthesize compounds such as SmFe11Ti or SmFe10Si2 seriously underperform in magnetically hard alloys. It is not known yet if they are predisposed to specific lattice defects, and little attention has been paid to this issue so far. It is possible that the infiltration treatment reported in Ref. [29], which is similar to the one that proved to be so effective in improving the Nd-Fe-B magnets, will be the solution.
《Acknowledgements》
Acknowledgements
This work was supported by the US Department of Energy, United States (DE-FG02-90ER45413), EU Horizon 2020 Program (686056–NOVAMAG) and Ford Motor Company, United States.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
G.C. Hadjipanayis, A.M. Gabay, A.M. Schönhöbel, A. Martín-Cid, J.M. Barandiaran, and D. Niarchos declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




