《1. Introduction》
1. Introduction
Groundwater treatment sludge is an iron (Fe)-containing waste from groundwater treatment plants that is produced in large quantities as a waste product of potable water production [1,2]. The sludge contains fine particles and is rich in Fe and manganese (Mn) oxides [3]. Traditionally, the sludge is disposed of as waste by discharging it into deep wells and/or waterways, which may lead to the run-off of Fe/Mn into nearby waters [4]. Given tightening solid waste disposal regulations, groundwater treatment plants generally handle the sludge by mechanical dewatering and harmless solidification before sending it to landfills. However, this process is costly and complicated [5].
Recycling of Fe and Mn from the sludge is an optional strategy, but the addition of exogenous acid and Mn reagent [6,7] limits its application. In recent years, the sludge has been directly applied to adsorb phosphate and heavy metals, such as Ni2+ [7], Cu2+, Zn2+, and Cd2+ [8], in wastewater. After adsorption, the multistep separation process of the sludge from water includes tedious centrifugation, coagulation, and/or filtration [4,8]. Conversion of the weakly magnetized sludge into magnetic adsorbent (MA) may be an alternative option to rapidly separate the sludge from water.
Fe oxides in the sludge can be converted to magnetite by heating over 600 C with the addition of coal [9] and iron(II) sulfide (FeS), or the injection of hydrogen (H2) [10] and methane (CH4) [11]. The conversion can also be performed under a mild temperature of 160 °C via a facile hydrothermal method with ethylene glycol [3] or ascorbic acid [5] as a reductant. With the addition of reductant, the Fe3+ in the sludge is reductively dissolved into Fe2+ and then reoxidized to form magnetite and/or maghemite under different conditions [3]. Without the addition of reductant, the reductive dissolution of Fe oxides is interrupted [5,6]. As a result, the product is weakly magnetized.
A synthesized low-cost adsorbent produced from waste, with a good magnetic response [12], has been shown to have promising application in wastewater treatment [13]. The surface functional group ( Fe–O–H) of the MA exhibited good adsorption capacities for both cationic and anionic contaminants in wastewater [14–16], especially for heavy metals and negatively charged colloid. In addition, the MA can be applied to the synthesis of carbon and/or silicon material [17,18] to enhance their magnetic separation after use [19,20].
Fe–O–H) of the MA exhibited good adsorption capacities for both cationic and anionic contaminants in wastewater [14–16], especially for heavy metals and negatively charged colloid. In addition, the MA can be applied to the synthesis of carbon and/or silicon material [17,18] to enhance their magnetic separation after use [19,20].
This study reports the development of a new approach to convert sludge to MA without the addition of reductant. The capacity of the synthesized adsorbent is evaluated by adsorption of the positively charged contaminant tetracycline (TC).
《2. Materials and method》
2. Materials and method
《2.1. Groundwater treatment sludge》
2.1. Groundwater treatment sludge
Groundwater treatment sludge was acquired from a drinking water treatment plant in Panshi City in Jilin Province, China. The sludge was vacuum dried at 50 °C overnight and then subjected to wet chemical analysis following the method of Sandroni and Smith [21]. The major elements of the sludge based on the dry weight were Fe (33.2%), silicon (Si, 6.2%), Mn (4.9%), calcium (Ca, 2.1%), aluminum (Al, 1.4%), potassium (K, 0.3%), and magnesium (Mg, 0.2%).
《2.2. Synthesis of magnetic adsorbent》
2.2. Synthesis of magnetic adsorbent
The synthesis of the MA from the groundwater treatment sludge was conducted as follows: 1 g of dried sludge, 20 mL of deionized water, and 10 mL of NaOH solution were placed in a 50 mL conical flask and stirred at 250 r·min-1 for 5 min, and then transferred into a 50 mL Teflon kettle. The kettle was heated at 160 °C for 10 h and then cooled to room temperature. The precipitate was collected and removed from the kettle by a magnet and then washed five times with deionized water, followed by vacuum drying overnight. In the experiment, the concentration of the NaOH solution was variably set at 0.6, 1, 2, and 4 mol·L-1 , respectively, and the obtained MAs were denoted as “MAx” (where x represents the NaOH concentration).
《2.3. Characterization of adsorbent》
2.3. Characterization of adsorbent
The X-ray powder diffraction (XRD) patterns of the sludge and MA were determined using a diffractometry system (RAPID-S; Rigaku, Japan) with copper (Cu)  radiation in the
radiation in the 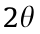 range of 20°–50°. The crystalline phase of the Fe oxides in the sludge and MA was investigated with a transmission Mössbauer spectrometer (MS-500; Oxford Instruments, UK) at room temperature. Magnetic measurement was carried out at room temperature by magnetometry (SQUID-VSM; Quantum Design, USA) with a SQUID-VSM system. The composition of the MA was determined following the method of the sludge described in Section 2.1. The valence states of Mn on the sludge and MA surface were determined by X-ray photoelectron spectrometry (XPS, VG-ADES; Thermal VG, UK) with Mg
range of 20°–50°. The crystalline phase of the Fe oxides in the sludge and MA was investigated with a transmission Mössbauer spectrometer (MS-500; Oxford Instruments, UK) at room temperature. Magnetic measurement was carried out at room temperature by magnetometry (SQUID-VSM; Quantum Design, USA) with a SQUID-VSM system. The composition of the MA was determined following the method of the sludge described in Section 2.1. The valence states of Mn on the sludge and MA surface were determined by X-ray photoelectron spectrometry (XPS, VG-ADES; Thermal VG, UK) with Mg  radiation. The morphologies of the sludge and of the MA were recorded by scanning electron microscopy (SEM, NanoSEM 450; FEI Co., USA).
radiation. The morphologies of the sludge and of the MA were recorded by scanning electron microscopy (SEM, NanoSEM 450; FEI Co., USA).
《2.4. Titration experiment》
2.4. Titration experiment
The total surface site concentrations (Hs) of the MA were determined by a potentiometric titration method [5]. First, 0.2 g of MA was added to 50 mL deionized water and then suspended by nitrogen (N2) bubbling. Second, the pH of the solution was titrated to 3 with 0.2 mol·L-1 HCl and then back-titrated to 11 with 0.2 mol·L-1 NaOH. The control was performed using a blank solution without MA. During titration, the functional sites on the MA surface were occupied by metallic cations and H+ through the following possible reactions [22]:

where SO- represents the surface sites, such as
SO- represents the surface sites, such as  FeO-,
FeO-,  SiO-, and
SiO-, and  AlO-; and Me represents the metallic cations, such as Na+ , Mg2+, and Ca2+.
AlO-; and Me represents the metallic cations, such as Na+ , Mg2+, and Ca2+.
Third, the Gran function value (G) was calculated using Eqs. (3) and (4):

where  represent the volumes (mL) of the deionized water, the titrated HCl, and the NaOH, respectively.
represent the volumes (mL) of the deionized water, the titrated HCl, and the NaOH, respectively.
By drawing the Gran plot, the equivalence points  were calculated from the intersections of the linear portions with the G value versus the NaOH volume.
were calculated from the intersections of the linear portions with the G value versus the NaOH volume.
Fourth, the Hs (mmol·g-1 ) of the MA was calculated using the following equation:

where CNaOH represents the concentration of NaOH (mol·L-1 ) and m1 represents the weight of the MA (g). The superscript MA and blank represent the test sample with the addition of MAs and the blank sample without adding MAs, respectively.
《2.5. Batch adsorption experiment》
2.5. Batch adsorption experiment
Of the four MAs, MA2 demonstrated the optimal saturation magnetization (8.15 emu·g-1 ) and Hs (0.75 mmol·g-1 ), and was used for subsequent TC adsorption. 30 mg·L-1 of TC stock solution was used to investigate the effect of pH on MA2 adsorption; the pH range was from 3 to 11, and was adjusted by adding 5% HCl or 5% NaOH. 0.1 g MA2 was mixed with 20 mL stock solution in a 50 mL flask. The flask was placed in a shaker (THZ-98A, Yiheng, China) and shaken at 150 r·min-1 for 30 h at room temperature. Next, MA2 was magnetically removed from the flask and the supernatant was collected for pH and TC analysis. The adsorption capacity (qe, mg·g-1 ) of the TC at equilibrium was calculated by the following equation:

where C0 and Ce are the initial and the equilibrium concentrations of TC (mg·L-1 ), respectively; V is the solution volume (L); and m2 is the weight of the MA2 (g).
The adsorption kinetics of TC on MA2 were investigated at pH 5 with a TC concentration of 30 mg·L-1 . Equilibrium isotherm experiments were also performed at pH 5 with a TC concentration ranging from 0 to 2000 mg·L-1 and an equilibrium time of 30 h.
All adsorption experiments were conducted three times, and the experimental data were averaged.
《3. Results and discussion》
3. Results and discussion
《3.1. Fe oxide phase transformation》
3.1. Fe oxide phase transformation
The transmission Mössbauer spectroscopy and XRD experiments were performed to demonstrate the phase transformation of Fe oxides in the sludge after hydrothermal treatment. As shown in Fig. 1, the XRD pattern of the sludge presented a weak peak 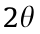 = 33.1, which belonged to hematite (JCPDS No. 33-0664). The Mössbauer spectrum of the sludge (Fig. 2(a)) showed a strong doublet and a weak sextet. The doublet exhibited an isomer shift (IS) of 0.26 mm·s-1 and an electric quadrupole splitting (QS) of 0.71 mm·s-1 , and was attributed to ferrihydrite. The sextet spectrum of IS = 0.41 mm·s-1 and QS = 0.36 mm·s-1 was affiliated with hematite. After calculating the relative area of the subspectrum, the ferrihydrite content was determined to be approximately 91.2% in the Fe oxides of the sludge (Supplementary data, Table S1), indicating that it is predominant in the sludge.
= 33.1, which belonged to hematite (JCPDS No. 33-0664). The Mössbauer spectrum of the sludge (Fig. 2(a)) showed a strong doublet and a weak sextet. The doublet exhibited an isomer shift (IS) of 0.26 mm·s-1 and an electric quadrupole splitting (QS) of 0.71 mm·s-1 , and was attributed to ferrihydrite. The sextet spectrum of IS = 0.41 mm·s-1 and QS = 0.36 mm·s-1 was affiliated with hematite. After calculating the relative area of the subspectrum, the ferrihydrite content was determined to be approximately 91.2% in the Fe oxides of the sludge (Supplementary data, Table S1), indicating that it is predominant in the sludge.
《Fig. 1》

Fig. 1. XRD patterns of the sludge, MA0.6, MA1, MA2, and MA4.
With the addition of NaOH, the diffraction peak of the hematite became sharp, and a new peak appeared in the curve of MA0.6 at 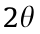 = 35.6, which belonged to maghemite (JCPDS No. 39-1346). Accordingly, three subspectra were observed in the Mössbauer spectrum of MA0.6 (Fig. 2(b)), two of which belonged to ferrihydrite and hematite, respectively, and the third, a new subspectrum with IS = 0.32 mm·s-1 and QS = 0.23 mm·s-1 , being attributed to maghemite.
= 35.6, which belonged to maghemite (JCPDS No. 39-1346). Accordingly, three subspectra were observed in the Mössbauer spectrum of MA0.6 (Fig. 2(b)), two of which belonged to ferrihydrite and hematite, respectively, and the third, a new subspectrum with IS = 0.32 mm·s-1 and QS = 0.23 mm·s-1 , being attributed to maghemite.
《Fig. 2》

Fig. 2. Mössbauer spectrum of (a) sludge, (b) MA0.6, (c) MA1, (d) MA2, and (e) MA4.
With the NaOH concentration increasing from 0.6 to 4 mol·L-1 , the diffraction peaks of hematite and maghemite intensified (Fig. 1). Moreover, the percentages of hematite and maghemite increased from 9.3% to 17% and from 2.1% to 13.3%, respectively, whereas the percentage of ferrihydrite steadily decreased from 88.6% to 69.7% (Supplementary data, Table S1). These results indicate that the ferrihydrite in the sludge was transformed to maghemite and hematite. Pure ferrihydrite was converted rapidly to hematite when the temperature was higher than 150 °C [23], but the transformation proceeded slowly in the presence of impurities such as Si, Al, and phosphorus (P) [23,24]. Liu et al. [25] reported that the product of phosphate ferrihydrite with a P/Fe ratio of 0.03 at 150 °C for 120 d was a mixture of maghemite, hematite, and residual ferrihydrite. Similar mixtures containing the same three Fe oxides were also identified with the Mössbauer spectrum in the MA (Fig. 2). Sidhu [26] reported that maghemite was completely transformed to hematite after heating at 500 °C for 3 h, and was retarded in the presence of impurities (e.g., Si and Al). Thus, in the sludge, the transformation of ferrihydrite to hematite was a two-step process, with maghemite being intermediate.
The transformation of ferrihydrite was considerably promoted by increasing NaOH concentration. With addition of NaOH, dissolution of the Si/Al oxides in the sludge occurred. As shown in Fig. 1, quartz and albite were the well-crystallized Si/Al oxides in the sludge, and these diffraction peaks gradually disappeared as the NaOH concentration increased to 4 mol·L-1 . These results indicated the dissolution of Si/Al oxides. The Si/Fe and Al/Fe ratios in the sludge were 0.37 and 0.09, respectively, and dropped to 0.17 and 0.04 (Fig. 3) in MA4. Accordingly, the optimal transformation of ferrihydrite was also observed in MA4 (Fig. 2 and Table S1). This phenomenon demonstrated that the loss of Si/Al impurities in the sludge promoted ferrihydrite transformation. Sludge is rich in ferrihydrite, which exists in a tetrahedrally coordinated iron structure (i.e., the iron ions are coordinated by six oxygen/hydroxyl groups) [27]. The hydroxyl groups exhibit coordinated unsaturation after dehydroxylation at high pH, thereby serving as the linkage of the small ferrihydrite particles, and allowing them to aggregate in the form of large hematite particles [28]. However, the impurities in the sludge, such as Si and Al, associate with the unsaturated sites to form an Fe–O–M (where M represents Si and Al) surface layer, which blocks the linkage of small ferrihydrite particles and inhibits ferrihydrite transformation [29]. When NaOH is introduced into the hydrothermal process, the Si/Al species with the probable forms of  [28,30,31] and
[28,30,31] and  , respectively, on the unsaturated sites are replaced by OH via ion exchange [32]. This phenomenon results in a low ratio of Si/Fe and Al/Fe in the MA and promotes the ferrihydrite transformation.
, respectively, on the unsaturated sites are replaced by OH via ion exchange [32]. This phenomenon results in a low ratio of Si/Fe and Al/Fe in the MA and promotes the ferrihydrite transformation.
《Fig. 3》

Fig. 3. Molar ratios of Mn, Si, Al, and Na to Fe in the sludge and MAs.
The content of Mn in the sludge was lower than that of Si and could be coordinated to the unsaturated sites of ferrihydrite to form an Fe–O–Mn layer. Unlike the Si/Fe and Al/Fe ratios, the Mn/Fe ratio in the sludge was 0.16 and remained almost constant before and after hydrothermal treatment (Fig. 3) due to the insolubility of Mn at high pH. The valence state of Mn in the sludge and MA was characterized by XPS. As shown in Fig. 4, the spectra of the sludge had an asymmetric peak (peak A) of Mn 2p3/2 at 641.5 eV and a weak peak (peak B) at 646.3 eV, which matched well with the Mn4+ in MnO2 [33] and the Mn2+ in the Mn–O bond[34], respectively. When the NaOH concentration increased to 1 mol·L-1 , peak B disappeared, indicating oxidization of Mn2+ by the dissolved oxygen in the liquid phase [35]. During Mn2+ oxidization, the Fe3+ in ferrihydrite served as an electron transporter, accepting electrons from Mn2+ and transferring them to oxygen (O2) [36]. The reductive dissolution and recrystallization of ferrihydrite occurred at this stage, resulting in the formation of maghemite and hematite.
《Fig. 4》

Fig. 4. High-resolution Mn 2p XPS spectrum of iron sludge and MAs.
《3.2. Magnetic separation》
3.2. Magnetic separation
The magnetic property of the MA was significantly correlated with the percentage of maghemite in the MA. As shown in Fig. 5, a weak magnetic response was demonstrated by the sludge because no maghemite was present in it. Magnetization appeared with the hydrothermal treatment in the presence of NaOH, due to the transformation of ferrihydrite to maghemite. When the NaOH concentrations were 0.6, 1, 2, and 4 mol·L-1 , the saturation magnetizations of the synthesized MA were 0.43, 1.1, 8.2, and 10.9 emu·g-1 , respectively; this finding was in agreement with the change in the maghemite percentage (Fig. 2 and Table S1).
《Fig. 5》

Fig. 5. Magnetic hysteresis curves of the sludge, MA0.6, MA1, MA2, and MA4. H: magnetic field strength. 1 Oe = 79.5775 A·m-1 .
《3.3. Morphology》
3.3. Morphology
SEM images of the sludge and MA are shown in Fig. 6. The sludge was composed of amorphous aggregates covered with small particles ranging from 200 to 500 nm in size. After hydrothermal treatment, the surface of the MA became coarse (Figs. 6(b, c)), which was attributed to the dissolution of Si/Al oxides in the presence of NaOH. The degree of roughness increased with elevation of the pH.
《Fig. 6》
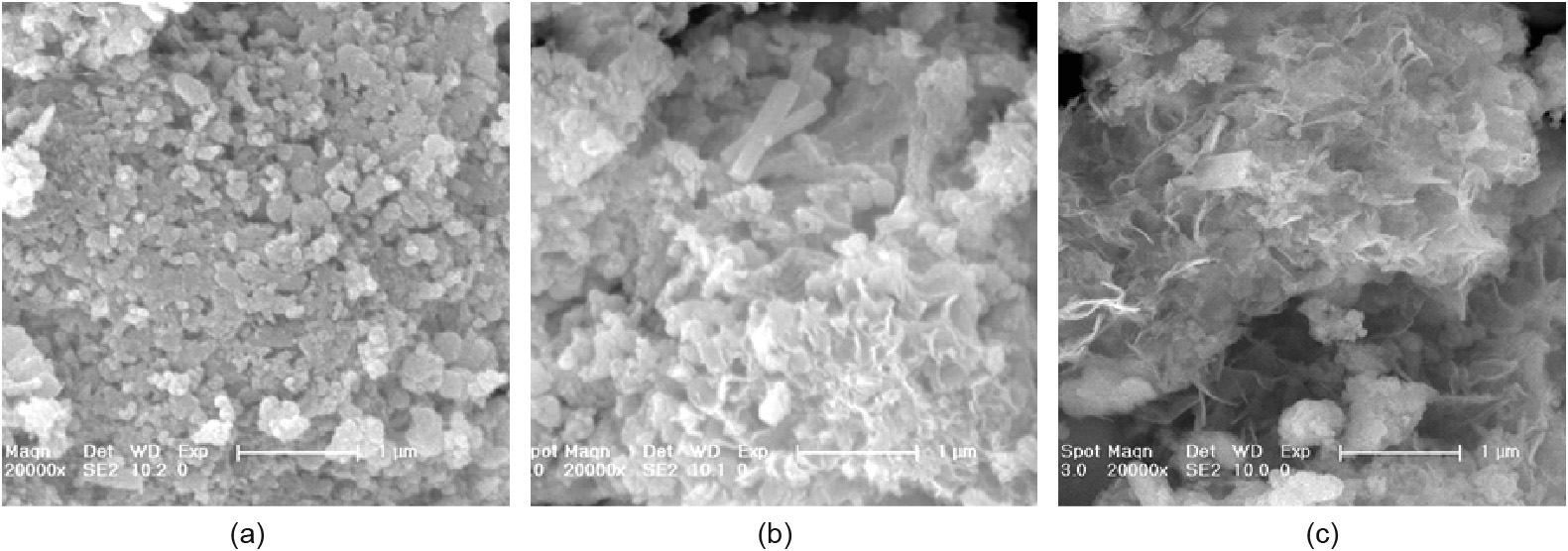
Fig. 6. SEM images of (a) the sludge, (b) MA1, and (c) MA2.
《3.4. Adsorption》
3.4. Adsorption
The Hs was an important parameter for estimating the adsorption property of the MA. Calculating from Fig. 7, the Hs of the MA generally declined from 1.03 mmol·g-1 for MA0.6 to 0.96 mmol·g-1 for MA1, 0.75 mmol·g-1 for MA2, and 0.51 mmol·g-1 for MA4 as the NaOH concentration increased. This decreasing trend was similar to that shown by the ferrihydrite percentage in the MA. The surface sites of the MA give it the ability to coordinate cations, such as Mg2+, Ca2+, and Na+ . In the hydrothermal process, Na+ was integrated into the coordination sites; thus, the Na/Fe molar ratio decreased from 0.13 for MA0.6 to 0.11 for MA2 and 0.09 for MA4 (Fig. 3), even with the increase in NaOH concentration. This decreasing trend was in agreement with the decreases in Hs and in the ferrihydrite percentage. Ferrihydrite was predominant in the sludge and had an average coordination site number of 5.4, which was higher than that of the Fe oxides (e.g., hematite) with high crystallinity [28]. Thus, phase transformation of ferrihydrite was an indicator of MA with a low Hs. Sajih et al. [37] reported that the adsorption of radium on newly generated ferrihydrite particles was nearly 100% and decreased to approximately 20% after ferrihydrite was transformed to goethite and hematite. MA2 had an Hs of 0.75 mmol·g-1 and a saturation magnetization of 8.2 emu·g-1 , indicating that MA2 is a desirable adsorbent with the property of magnetic separation.
《Fig. 7》

Fig. 7. Gran plots of MA0.6, MA1, MA2, and MA4.
The adsorption property of MA2 was investigated using TC as a target. Fig. 8 shows the effect of the initial pH on MA2 adsorption for TC. The removal rate of TC was approximately 87% at an initial pH below 9 and decreased to 26% at an initial pH of 11, indicating that TC adsorption on MA2 is pH dependent. When the initial pH was lower than 9, the final pH was lower than 7.4; thus, the TC was in cationic form due to the loss of protons from the phenolic diketone moiety [38]. The cationic TC could associate with the coordination sites of MA2, and an exchange between the TC and the coordinated cations (e.g., Na+ ) occurred [39], resulting in a high removal rate of TC. When the initial pH increased to 11, the final pH increased to 8.5; in this case, the TC presented as a monovalent anion due to the loss of protons from the tricarbonyl system and the phenolic diketone moiety [38]. Therefore, the cationic exchange on the MA2 surface was interrupted, resulting in a low TC removal rate.
《Fig. 8》
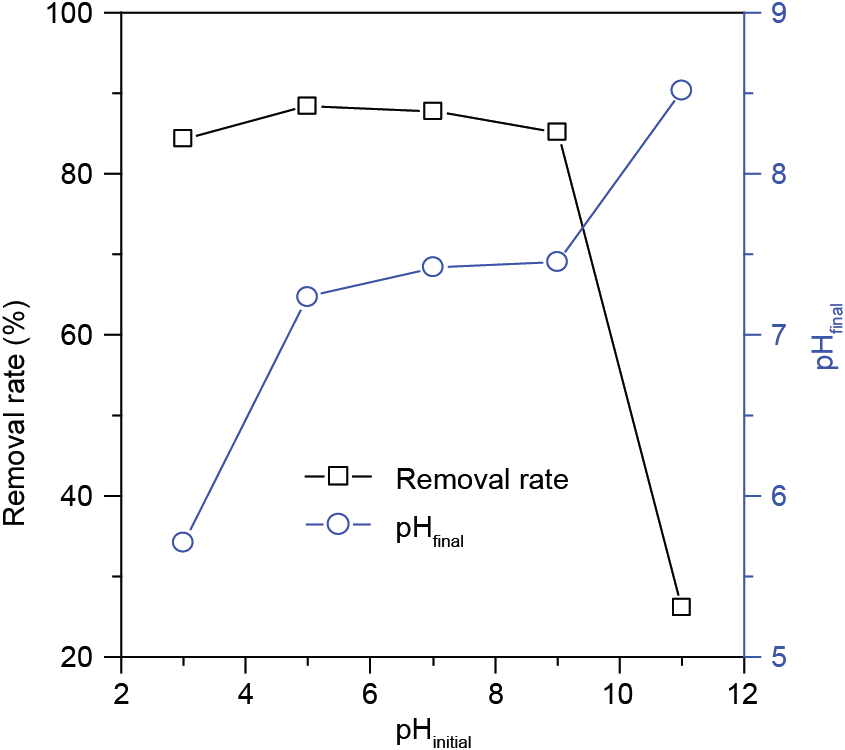
Fig. 8. The removal rate of TC and the final pH in the initial pH range of 3–11.
The adsorption kinetics of TC on MA2 were investigated, and the adsorption data was fitted with pseudo-first-order and pseudo-second-order models (Figs. 9 (a, b)). The equation of the pseudo-first-order kinetics is as follows:

where qt is the adsorption capacity (mg·g-1 ) of the TC at any instant time, t ; and k1 is the rate constant (L·h-1 ) of the pseudo-first-order model.
《Fig. 9》

Fig. 9. Linear fitted curve of the three models for TC adsorption. (a) Pseudo-first-order kinetic model; (b) pseudo-second-order kinetic model; (c) intraparticle diffusion model.
The equation of the pseudo-second-order kinetics is as follows:

where k2 is the rate constant (×103 g·(mg·h)-1 ) of the pseudosecond-order model.
Table 1 provides the kinetics parameters and their values of different kinetics models. The experimental qe fitted well with the calculated value of the pseudo-second-order model, but not with that of the pseudo-first-order model. The results suggest that valence forces were involved in the exchange of electrons between TC and MA2, and that chemisorption might be the mechanism for the adsorption of TC on MA2 [40].
《Table 1》
Table 1 Parameters and their values of the three kinetics models.

R2 is the regression coefficient.
Weber’s intraparticle diffusion model was used to analyze the adsorption data and was expressed as follows:

where k3 represents the intraparticle diffusion rate constant (mg· (g·h1/2) -1 ) and C represents the constant related to the boundary layer effect of the adsorption.
As shown in Fig. 9(c), Weber’s model had two portions. The first portion corresponded to the gradual adsorption stage governed by intraparticle diffusion, and the second portion corresponded to the final equilibrium stage, where the intraparticle diffusion starts to slow down due to the extremely low TC concentration in the solution [41,42]. The first portion did not pass through the zero point, which indicated that the intraparticle diffusion was the ratecontrolling step.
As shown in Fig. 10(a), the adsorption isotherm of the TC on MA2 was investigated. The adsorption data were analyzed using non-linear Langmuir (Eq. (10)) and Freundlich equations (Eq. (11)).

《Fig. 10》
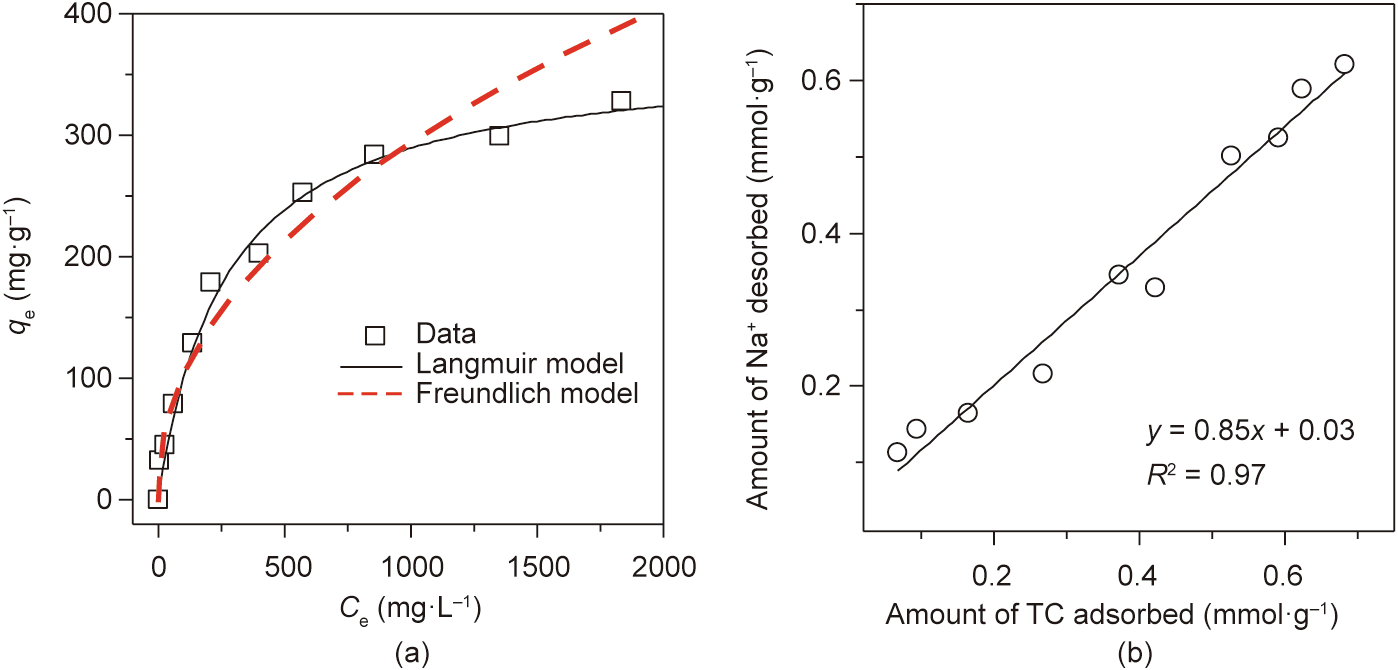
Fig. 10. (a) Adsorption isotherm of TC on MA2; (b) amount of Na+ desorbed as affected by TC adsorption on MA2.
where qm is the maximum adsorption capacity (mg·g-1 ), KL is the Langmuir constant (L·mg-1 ), KF is the Freundlich constant (mg1-1/n ·L1/n ·g-1 ), and n is related to the adsorption intensity [30].
Table 2 lists the parameters and their values of the Langmuir and Freundlich models. The adsorption data was in good agreement with the Langmuir model, with a high regression coefficient value (R2 ) of 0.996, in comparison with the Freundlich model. The results indicate that the adsorption of TC on MA2 was of a monolayer type [43,44]. The maximum capacity of MA2 for TC adsorption was 362.3 mg ·g-1 , which was lower than those of costly carbon materials [45], but apparently higher than those of other synthesized MAs (Table 3) [45–53], such as magnetic polyacrylonitrile nanofiber mat [46], magnetic resin [47], and halloysite/ CoFe2O4 composites [48]. Therefore, MA2 has great potential for TC adsorption. The amount of Na+ desorbed from the MA2 showed a positive correlation with the amount of TC adsorbed on the MA2 (Fig. 10(b)), which confirmed that cationic exchange was the major mechanism for TC adsorption on MA2.
《Table 2》
Table 2 Parameters and their values of the different isotherm models.

《Table 3》
Table 3 Comparison of the adsorption capacity of TC on MA2 with other adsorbents.

《3.5. Environmental application》
3.5. Environmental application
A facile hydrothermal method for the synthesis of MA was developed by treating groundwater treatment sludge, but this method is not feasible for red mud. The weakly crystallized ferrihydrite in the sludge was transformed into well-crystallized maghemite, and the prepared adsorbent had a good magnetic response. In comparison with the sludge, red mud is only rich in well-crystallized hematite and andradite [10], which need to be reductively dissolved by the addition of reductant with the formation of magnetic species [11]. A non-reductive method for MA preparation would save the disposal cost of the sludge. The MA exhibited a desirable adsorption capacity for TC and other cationic contaminants [54,55], and was reusable after regeneration under alkaline condition [56]. At the end of five cycles, the removal rate of TC was 60.9%, which was slightly lower than the 63.2% of the first round. These advantages demonstrate that the prepared MA has potential application in environment pollution control.
《4. Conclusions》
4. Conclusions
With alkaline hydrothermal treatment, groundwater treatment sludge comprising a mixture of ferrihydrite and other impurities (e.g., Si, Al, and Mn) was converted into MA in three steps: ① dissolution of Si/Al oxides in the sludge to the liquid fraction, ② oxidization of residual Mn2+ by dissolved oxygen with ferrihydrite under catalysis, and ③ promotion of the phase transformation of ferrihydrite to maghemite and hematite. The ideal MA2, synthesized with 2 mol·L-1 NaOH, exhibited a strong saturation magnetization of 8.2 emu·g-1 and a high adsorption capacity of 362.3 mg·g-1 for TC. Cationic exchange was the major mechanism for MA2 adsorption of TC. The results indicate that MA2 from recycled sludge is a promising adsorbent for the removal of TC from wastewater.
《Acknowledgements》
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51578118, 51678273, 51878134, and 51878133), the Fundamental Research Funds for the Central Universities (2412017QD021) and the Science and Technology Program of Jilin Province (20190303001SF).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Zhan Qu, Yaqiong Wu, Suiyi Zhu, Yang Yu, Mingxin Huo, Leilei Zhang, Jiakuan Yang, Dejun Bian, and Yi Wang declare that they have no conflicts of interest or financial conflicts to disclose.
《Appendix A. Supplementary data》
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eng.2019.06.001.














 京公网安备 11010502051620号
京公网安备 11010502051620号




