《1. Introduction 》
1. Introduction
2,4-Dinitroanisole (DNAN) is an important constituent of insensitive munitions that is intended to replace 2,4,6-trinitrotoluene (TNT) in munitions formulations. It is also used for the synthesis of dyes and insecticides [1]. Therefore, the production, transport, storage, and use of DNAN is expected to increase, which will result in large amounts of wastewater containing DNAN.
Accordingly, increased concern has been raised regarding the environmental fate and impacts of DNAN. DNAN is poorly soluble in water (about 276 mg·L-1 at room temperature), and produces rod-shaped crystals at higher concentrations, with adsorption to clay and organic soils [2]. DNAN has been found to be toxic to aquatic organisms, such as algae, cladocerans, and fish, and lethal to earthworms in soil [3–5]. Studies on rats have indicated that the acute toxicity of DNAN (lethal dose (LD50) = 199 mg·kg-1 ) could be even higher than that of TNT (LD50 = 794–1320 mg·kg-1 ) [6]. Moreover, the degradation products of DNAN, such as 2,4-dinitrophenol and nitrite, are even more toxic than DNAN itself. Furthermore, DNAN can result in a persistent yellow color in wastewater, which must be removed by a pretreatment process.
Only a few studies have been conducted to characterize the environmental fate and behavior of DNAN, and to examine the biological transformation of DNAN under aerobic and anaerobic conditions. In this research, the relevant sensitivity, chemical compatibility, and thermal properties of DNAN have been determined through a literature review and experimental work [6].
Advanced oxidation processes (AOPs) appear to be some of the most promising methods for the treatment of wastewater containing nitroaromatic compounds. AOPs are associated with the generation of radical species (e.g., hydroxyl radical (·OH)), which, given sufficient contact time and proper conditions, can mineralize the target pollutants to carbon dioxide (CO2) [7]. Ultraviolet (UV) photolysis in combination with hydrogen peroxide (H2O2) oxidation is an important AOP technology that has been used to treat wastewater contaminated with nitroaromatic compounds [8]. H2O2 is readily available and relatively inexpensive, and the photolysis of H2O2 results in the generation of ·OH, which may degrade contaminants. UV/H2O2 has been shown to be an effective degradation and mineralization treatment for most organic compounds [9–13].
Thus far, studies have examined the degradation of DNAN using the Fenton oxidation process [1], biotic or abiotic transformation [14–18], alkaline hydrolysis [19,20], and bimetal reductive treatment [21]. Only a few studies have been conducted on the oxidation of DNAN with UV/H2O2 [22], and the results have shown that photo-oxidation is the main mechanism for DNAN degradation, with the formation of nitrite and nitrate as major nitrogen (N) species and 2,4-dinitrophenol as a minor species [22]. An evaluation of treatment technologies for industrial process wastewater containing insensitive munitions (i.e., DNAN) has been summarized [23], but the optimal conditions for the design and operating parameters for a UV/H2O2 degradation process need to be determined. The objective of this study was to investigate the feasibility of treating DNAN-contaminated water by means of UV/H2O2 advanced oxidation, and to determine the optimal oxidation conditions for DNAN degradation, including H2O2 dosage and initial pH. This study has fundamental implications for the treatment of wastewater containing DNAN.
《2. Materials and methods 》
2. Materials and methods
Aqueous DNAN stock solutions were prepared by dissolving reagent-grade DNAN (C7H6N2O5; 98% purity, Sigma Aldrich LLC., USA) in tap water. Considering that the solubility of DNAN in water at room temperature is about 260 ppm, the initial concentration of the DNAN solutions prepared in this study was about 250 ppm. The UV lamp used for the treatment was an LSE Lighting UV Bulb (254 nm, 13 W for Pura 1GPM 10–212 UV10 UV11). H2O2 solution (30%) was used in the oxidation process (reagent grade, Thermo Fisher Scientific Inc., USA). Sodium hydroxide (NaOH) was used to adjust the initial pH of the DNAN solutions (reagent grade, Thermo Fisher Scientific Inc., USA).
Oxidation experiments of DNAN by UV/H2O2 were conducted using a 1000 mL glass beaker. The 1000 mL beaker, which had a diameter of 8.9 cm and a height of 18.7 cm, was used as the reactor. The UV lamp was fixed in the center of the beaker, with most of it under the surface. A magnetic mixer with a length of 3 cm was used to mix the solutions. The UV intensity measured during the oxidation process was about 4500 lx. In order to determine the optimal conditions for the treatment of DNAN, batch experiments were conducted with different H2O2 dosages (1500, 2250, 3000, and 4500 ppm) at a fixed pH of 7 and at different initial pH (4, 5, 6, and 7) using a fixed H2O2 dosage (1500 ppm). For each experiment, 800 mL DNAN solution was used, and the initial concentration of DNAN was about 250 ppm, or 35 ppm as DNAN-N. Next, the solutions were exposed to the UV light for a total time of 9 h while being continuously mixed by magnetic mixers. Samples were collected (25 mL) from the reactor at specific time intervals during the degradation process for analysis. Data were checked for deviations from normality and homogeneity of variance before performing statistical analyses. Pearson pair-wise correlation coefficients between the N-concentrations and carbon (C)-concentrations were determined using SPSS software (Statistics 21, IBM, USA).
The analysis of DNAN was performed with a high-performance liquid chromatography (HPLC) system (Varian Inc., USA; equipped with a ProStar 410 Auto-sampler and a 330 UV–Vis PDA detector). A Dionex AcclaimTM 120 C18 column (15 μm, 4.6 mm × 250 mm, Thermo Fisher Scientific Inc., USA) was used. The eluent was an isocratic methanol–water mixture at a ratio of 70:30 (v/v). It was pumped at a flow rate of 1 mL·min-1 . The analytical wavelength was 284 nm and the sample injection volume of was 10 μL. Under these conditions, DNAN elutes at 4.9 min.
The total-N and nitrite-nitrogen (NO2-N) of the samples were analyzed using UV spectrophotometry (DR2800, Type LPG422.99.00012, 15 V, 30 VA, HACH, USA). The concentrations of H2O2 were measured using a H2O2 test kit (Model HYP-1, HACH, USA). The nitrate-nitrogen (NO3-N) concentrations of the samples were measured by means of an ion chromatography system (Dionex, Thermo Fisher Scientific Inc., USA) equipped with an anion separation column (AS16). Total organic carbon (TOC) and total carbon (TC) were measured using a Phoenix 8000 UV– Persulfate TOC analyzer, equipped with a TOC boat sampler (Rosemount Dohrmann Model 183), and TOC Talk software (Teledyne Tekmar Company, USA). A TOC and TC concentration range of 0.1–20 ppm C were used to calibrate the instrument.
《3. Results and discussion 》
3. Results and discussion
《3.1. Effect of H2O2 dosage on kinetics of DNAN removal》
3.1. Effect of H2O2 dosage on kinetics of DNAN removal
The kinetics of DNAN removal under four different H2O2 dosages is shown in Fig. 1. The DNAN removal rate depends markedly on H2O2 dosage, indicating that H2O2 dosage has a strong effect on the degradation rate of DNAN. For dosages of 1500– 3000 ppm, the degradation rate increased with increasing dosage. This is because the increasing H2O2 concentration can promote the formation of ·OH, which can accelerate the DNAN degradation process. However, studies on the photo-degradation of some organics have shown that excessive H2O2 may reduce the degradation rate because it acts as a scavenger for ·OH [24–26]. From Fig. 1, it can be seen that the DNAN removal rate at 4500 ppm H2O2 is slower than that at 3000 ppm, suggesting that excessive H2O2 reduces the DNAN degradation rate. It can be concluded that excessive H2O2 may compete with DNAN for reaction with ·OH, which would decrease the degradation rate of DNAN.
《Fig.1》

Fig. 1. Kinetics of DNAN removal under four different H2O2 dosages: (a) 1500 ppm, (b) 2250 ppm, (c) 3000 ppm, and (d) 4500 ppm. Initial DNAN concentration ≈ 250 ppm, initial pH ≈ 7, under 13 W UV light.
The results show that the optimal H2O2 dose for the oxidation of a DNAN solution with an initial concentration of 250 ppm is 3000 ppm. However, a higher H2O2 dosage would result in higher treatment costs in engineering applications, and the H2O2 remaining in the treated solution must be removed for environmental protection. Thus, it is of great importance to optimize the H2O2 dose for the treatment of DNAN solutions.
The results show that DNAN degradation follows zero-order kinetics under our experimental conditions; the rate constants are summarized in Table 1. The zero-order kinetics were different from the reported pseudo-first-order decay kinetics of DNAN under sunlight and UV only, under which the degradation process was much slower [22]. This difference was probably due to the presence of H2O2, which can accelerate the DNAN degradation process. Linear regression was developed for the DNAN degradation kinetics; the results are shown in Fig. 1. The points after the degradation was finished were not used in the linear regression.
《Table 1》
Table 1 Zero-order rate constants of DNAN oxidation kinetics (k0).
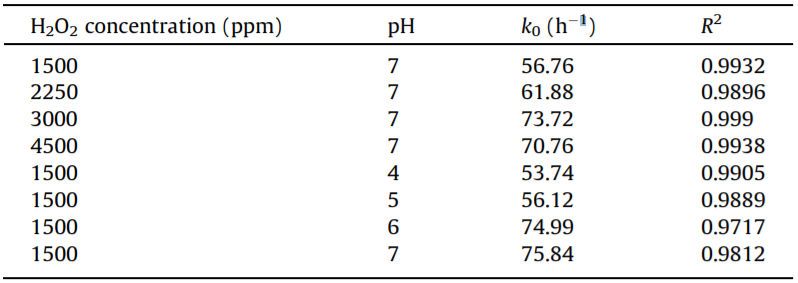
A lower H2O2 dosage of 750 ppm was used to treat the DNAN solution; the DNAN removal kinetics are shown in Fig. 2. It was clear that the kinetics of DNAN degradation were pseudo-firstorder decay kinetics. The results in Figs. 1 and 2 indicate that DNAN can easily be oxidized by UV/H2O2 in the presence of suffi- cient H2O2, and that the oxidation rate of DNAN is not affected by decreasing DNAN concentration during the treatment. Zeroorder decay kinetics for the advanced oxidation process for other organic contaminates, such as nitroguanidine, have also been reported [27].
《Fig.2》

Fig. 2. Kinetics of DNAN removal under an H2O2 dosage of 750 ppm. (a) Pseudofirst-order approach fitting result; (b) zero-order approach fitting result. Initial DNAN concentration ≈ 250 ppm, initial pH ≈ 7, 13 W UV light.
《Fig.3》

Fig. 3. Variation of H2O2 concentration and TOC during oxidation treatment of 250 ppm DNAN solution with 1500 ppm H2O2. Initial pH ≈ 7, 13 W UV light.
Fig. 3 shows the changes in H2O2 concentration and TOC during the DNAN oxidation process using 1500 ppm H2O2 for 9 h of treatment. While the DNAN degradation process was completed in about 5 h (Fig. 1), it took 9 h to reach about 95% TOC removal (Fig. 3). Considering that 1500 ppm H2O2 was completely consumed after 9 h of reaction, this dosage of H2O2 is suitable for the oxidation of DNAN solution with an initial DNAN concentration of 250 ppm.

Assuming that all the H2O2 was used to oxidize DNAN to nitrate (HNO3) and CO2, 730 ppm H2O2 was needed to completely oxidize 250 ppm DNAN, as calculated based on Eq. (1). Under experimental conditions, only part of the H2O2 was consumed to oxidize the DNAN and its degradation products, and the actual demand for H2O2 was greater than 730 ppm.
《3.2. Effect of initial pH on kinetics of DNAN removal 》
3.2. Effect of initial pH on kinetics of DNAN removal
In addition to H2O2 dosage, the effect of initial pH on DNAN degradation was studied. Batch experiments were conducted to treat DNAN solutions at an initial pH of 4, 5, 6, and 7, respectively, with the same H2O2 dosage of 1500 ppm. The kinetics of DNAN removal at four different initial pH values are shown in Fig. 4. The results show that DNAN degradation follows zero-order kinetics under the experimental conditions used here; the rate constants are shown in Table 1. Linear regression was developed for the DNAN degradation kinetics, and the results are shown in Fig. 4. The points after the degradation finished were not used in the linear regression.
《Fig.4》
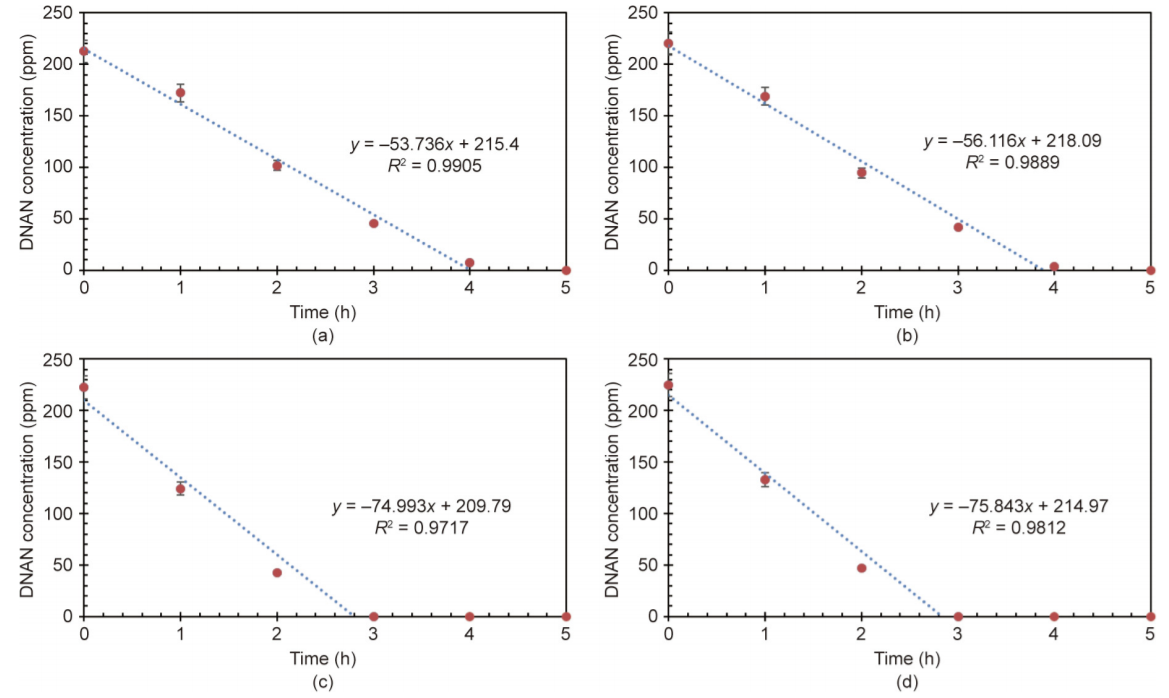
Fig. 4. Kinetics of DNAN removal at four different initial pH values: (a) pH = 4, (b) pH = 5, (c) pH = 6, and (d) pH = 7. Initial DNAN concentration ≈ 250 ppm, 1500 ppm H2O2, 13 W UV light.
Fig. 4 shows that the initial pH can affect the DNAN degradation processes to some extent: The higher the pH, the faster the degradation rate within the pH range of 4–7. When the pH is higher than 7, alkaline hydrolysis of DNAN will take place, which is a different degradation process [19,20]. However, the effect of initial pH on the degradation rate of DNAN is modest compared with the effect of the H2O2 dosage. Studies on the phototransformation rate of DNAN in water showed that a variation in the solution pH (pH 6.5–8.0) had only slight effects on the degradation rate of DNAN [22]. Considering that the initial pH of the DNAN solution is about 7, the most suitable pH is 7 for DNAN oxidation, as no pH adjustment is needed and the degradation efficiency is not affected.
Tables 2 and 3 show the changes in pH during the oxidation processes under different initial pH and H2O dosages. The results show that the pH decreased quickly when the oxidation process of DNAN began, regardless of the initial pH and H2O dosage, and remained constant at about 2 after the DNAN degradation processes finished. This finding indicates that some acid substances—mostly HNO3—were formed as a result of DNAN degradation.
《Table 2》
Table 2 Changes in pH during the DNAN degradation processes under different initial pH.

Initial DNAN concentration = 250 ppm, 1500 ppm H2O2, 13 W UV light.
《Table 3》
Table 3 Changes in pH during the DNAN degradation processes under different H2O2 dosages.
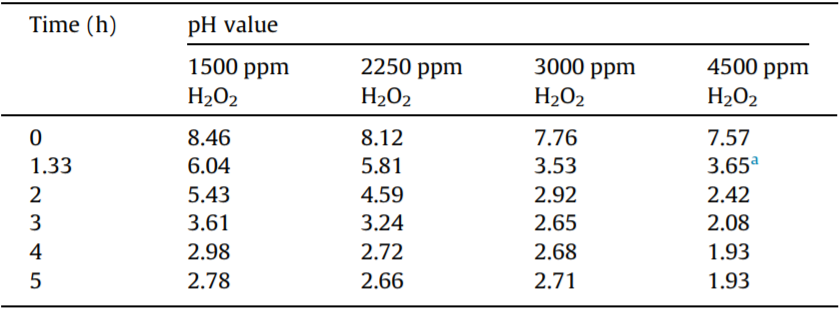
Initial DNAN concentration = 250 ppm, initial pH ≈ 7, 13 W UV light. a Measured at 1 h.
《3.3. N concentrations during the DNAN degradation processes 》
3.3. N concentrations during the DNAN degradation processes
In order to determine the oxidation products of DNAN, concentrations of the various kinds of N species—including total-N, ammonia, nitrate, and nitrite—formed during the oxidation processes of DNAN were measured in addition to the analysis of DNAN-N.
The results (Fig. 5) show that during the DNAN degradation processes, nitrate and nitrite were formed and no ammonia was detected. The concentration of NO2-N was very low and disappeared after the DNAN degradation processes finished, indicating that NO2-N is one of the major intermediates. Nitrite was oxidized to nitrate immediately after being generated from the decomposition of DNAN. The concentration of NO3-N continued to increase during the degradation processes, and the final concentration of NO3-N was similar to the initial DNAN-N concentration. This result suggests that NO3-N was the end product, and that almost all of the DNAN-N was transformed into NO3-N, similar to the other nitroaromatics treated with UV/H2O2 [8,28]. These results are consistent with those of other studies that have been conducted on the UV photolysis of DNAN [22], and suggest that photo-oxidation is a promising technique for the treatment of DNAN in wastewater.
《Fig.5》

Fig. 5. Variation of N compounds during the oxidation process of 250 ppm DNAN solution. Initial pH ≈ 7, 1500 ppm H2O2, 13 W UV light.
The total-N remained constant during the DNAN degradation process, indicating that no gaseous N was formed (Fig. 6). Furthermore, the N compounds showed similar change trends at a different initial pH within the range of 4–7. However, the DNAN-N decreased faster and the nitrate increased faster with a higher initial pH, as a higher pH increases the rate of the DNAN degradation progress. After 9 h of treatment, the NO3-N was still a little lower than the total-N, suggesting that there was a small portion of N remaining in the form of nitro-organics. Similarly, Fig. 7 shows that the TOC was about 5 ppm after 9 h of treatment, indicating that some organics containing N were present in the reacted solutions. This probably occurred because the very low concentration of H2O2 that remained after 9 h (Fig. 3) was unable to oxidize the residual nitro-organics into nitrate and CO2.
《Fig.6》
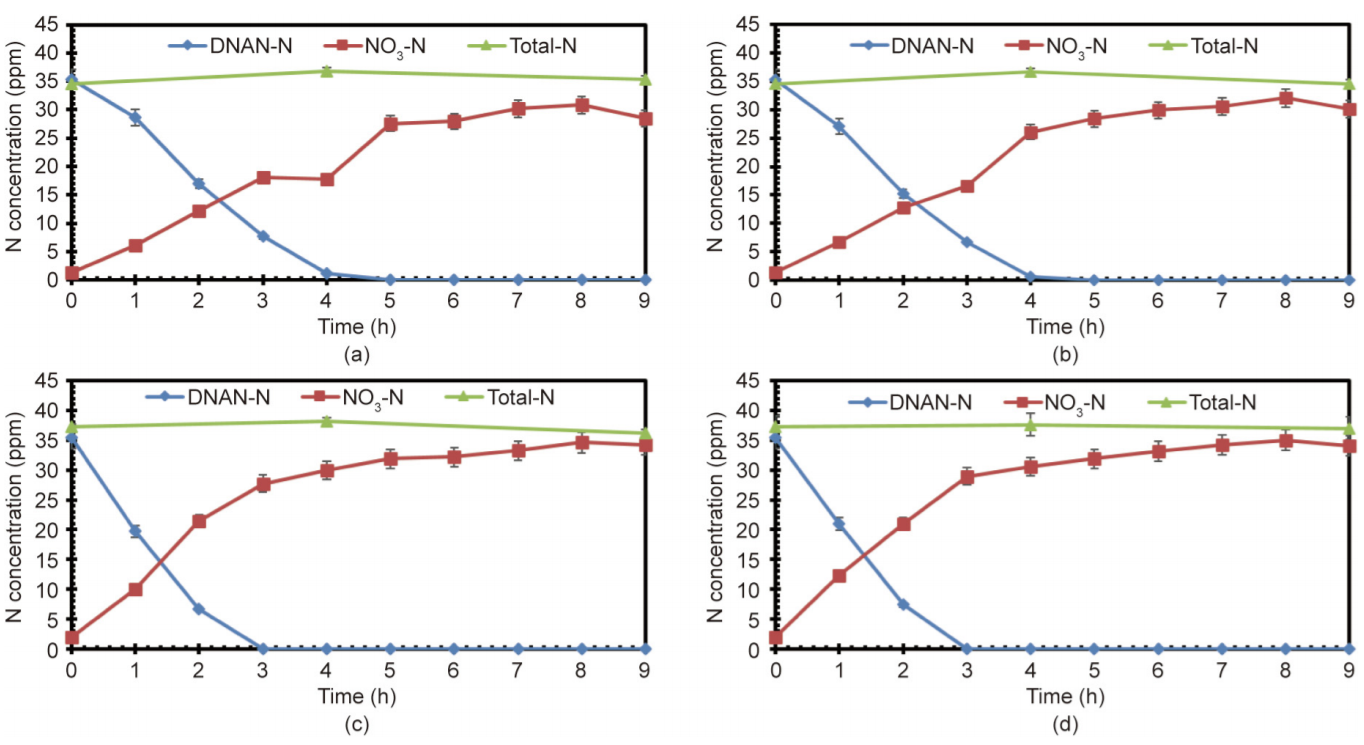
Fig. 6. Variation of N concentrations under different initial pH: (a) pH = 4, (b) pH = 5, (c) pH = 6 and (d) pH = 7. Initial DNAN concentration = 250 ppm, 1500 ppm H2O2, 13 W UV light.
《3.4. C concentrations during the DNAN degradation processes 》
3.4. C concentrations during the DNAN degradation processes
TOC and TC were measured during the degradation process of DNAN in order to investigate the changes of carbon species; the results are shown in Fig. 7. The TOC concentrations are almost the same as the TC concentrations, and both the TOC and TC decrease during the degradation process of DNAN. This result indicates that gaseous carbon (probably CO2) was generated during the DNAN degradation process, and that no carbonate or bicarbonate was present in the treated water. As discussed before, the pH decreased quickly during the oxidation processes when the oxidation process of DNAN began, regardless of the initial pH and the H2O2 dosage, and remained constant at about 2 after the DNAN degradation processes finished. This result indicates that some acid substances—mostly HNO3—were formed as a result of DNAN degradation, which also explains why no carbonate or bicarbonate was present in the treated water.
The results show that complete mineralization of DNAN can be achieved with UV/H2O2, as the TC and TOC decreased to about 5 ppm after 9 h of treatment. As the DNAN degradation finished in about 4 h, DNAN was converted to other organic compounds, which were subsequently degraded. The degradation process of DNAN may follow parts of the decomposition pathway of 2,4- dinitrotoluene by UV/H2O2, in which some lower molecular weight carboxylic acids are produced and then oxidized into CO2 [29]. This would explain why the pH increased slightly after the DNAN degradation was complete.
Fig. 6 shows that the rate of increase of nitrate was faster during the degradation process of DNAN than after the DNAN degradation finished, indicating that the cleavage of nitro groups took place in the early stages of DNAN decomposition. When the DNAN degradation was complete, about 77% of the N had been transformed into nitrate, suggesting that 23% of the N remained in the form of nitro-organics. Meanwhile, Fig. 7 shows that about 65% of the TOC remained at that time, which indicates that the nitrification process is faster than mineralization during DNAN degradation.
《4. Conclusions 》
4. Conclusions
The research results indicate that UV/H2O2 oxidation is a promising technique for the treatment of DNAN in solutions, as most of the DNAN can be oxidized to CO2 and nitrate. During the oxidation treatment, the total-N concentration remained almost constant, suggesting that no gaseous N compounds (i.e., nitric oxide, nitrous oxide, nitrogen gas, and ammonia) were formed. Nitrite was one of the major oxidation intermediates. The rate of DNAN oxidation was affected by the H2O2 dosage and pH. An H2O2 dosage of 1500 ppm and an initial pH of 7 were the optimal conditions for the treatment of 250 ppm DNAN solution.
《Acknowledgements》
Acknowledgements
The authors appreciate the insightful comments and constructive criticism of the independent peer reviewers and editorial board, which strengthened this manuscript.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Hailei Su, Christos Christodoulatos, Benjamin Smolinski, Per Arienti, Greg O’Connor, and Xiaoguang Meng declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




