《1. Introduction》
1. Introduction
Modern agribusiness plays a vital role in safeguarding and improving the production, quality, and quantity of food, feed, fiber, and fuel [1]. With its current offerings of insecticides, herbicides, fungicides, and biotechnology products, together with considerable investments in research and development, the global agribusiness industry contributes to growing public expectations for an adequate supply of high-quality food and agricultural sustainability. The industry is also addressing global challenges, such as population growth and rising caloric consumption, increasing environmental stresses across the globe, a changing regulatory landscape, and the development of resistance to existing active ingredients (AIs) and traits.
In recent years, increasing concerns over the impact of chemical pesticides on human health and the environment have stimulated the industry to search for alternative solutions, and have resulted in an increased quest for biological pest control agents [2–4]. The biopesticide market was valued at 2.83 billion USD in 2016, and is projected to reach 6.60 billion USD by 2022, at a compound annual growth rate (CAGR) of 15.43% during the forecast period [5].
The major categories of biologicals are:
• Microbials such as bacteria, viruses, protozoa viruses, or fungi, which are applied directly to plants;
• Macrobials, which are usually living organisms such as beneficial insects and nematodes;
• Semiochemicals such as pheromones;
• Plant extracts.
In addition to these, the RNA interference (RNAi) process has recently been identified as a very promising approach to complement the arsenal of foliar spray, soil, or seed treatments applied as chemical and biological crop-protection agents, and of plantincorporated protectants (PIPs). The right combination of improved cultivars, traits, and chemical and biological crop-protection products are essential to secure future sustainable farming.
《2. Technical background of RNAi》
2. Technical background of RNAi
RNAi is a natural mechanism that is present within all eukaryotes, including insects. It is driven by sequence-specific targeting, and results in the degradation of messenger RNA (mRNA) [6]. RNAi is believed to have evolved as a defense mechanism against viruses [7] and mobile genetic elements, such as transposons, that can affect the integrity of genomic DNA. The RNAi pathway is also employed in many organisms to regulate protein levels [8,9]. Since its discovery, RNAi has become a widely used laboratory tool to study gene function [10,11]. In addition to its use in cellular and genetic studies, the potential of harnessing RNAi as a molecular therapeutic and crop-protection agent has been highlighted [6,12–15]. Within insects, the RNAi process is triggered by the presence of double-stranded RNA (dsRNA) that is complementary to a specific mRNA. dsRNA can be synthesized within the cell or can come from an external source, in which case, it must be taken up by the cell (Fig. 1). Subsequently, the dsRNA is cleaved into short, 21–25 base pairs (bp) nucleotide fragments by a ribonuclease (RNase) named DICER [16]. These short RNA segments trigger mRNA degradation. These small RNA segments are further incorporated into the multi-protein complex named RNA-induced silencing complex (RISC) and are dissociated into single strands. One strand of the RNA duplex (the guide strand) associates with Argonaute, a protein with RNase activity, and binds to the complementary target mRNA by homologous base-pairing. Afterwards, Argonaute cleaves the bound mRNA, leading to its degradation. Because the entire process depends on precise complementary sequence recognition, only mRNAs containing stretches of homology with the relevant small interfering RNAs (siRNAs) are affected, this underpins the high selectivity of RNAi. In the absence of the appropriate mRNA, de novo synthesis of the corresponding protein ceases, and it is ultimately the lack of protein that leads to a loss-of-function phenotype. In certain insect species, RNAi effects can be triggered by the oral uptake of dsRNA, leading to systemic degradation of the corresponding mRNA, and resulting in a decreased pool of the corresponding protein product. In a situation where the protein product is crucial for the insect’s viability and survival, RNAi can lead to morbidity or death of the insect. This is the guiding principle behind the use of dsRNA for insect control and crop protection. The suitability of a target gene for insect control depends on its role in a crucial metabolic process, and on the critical threshold of expression and halflife of the corresponding protein product.
《Fig. 1》
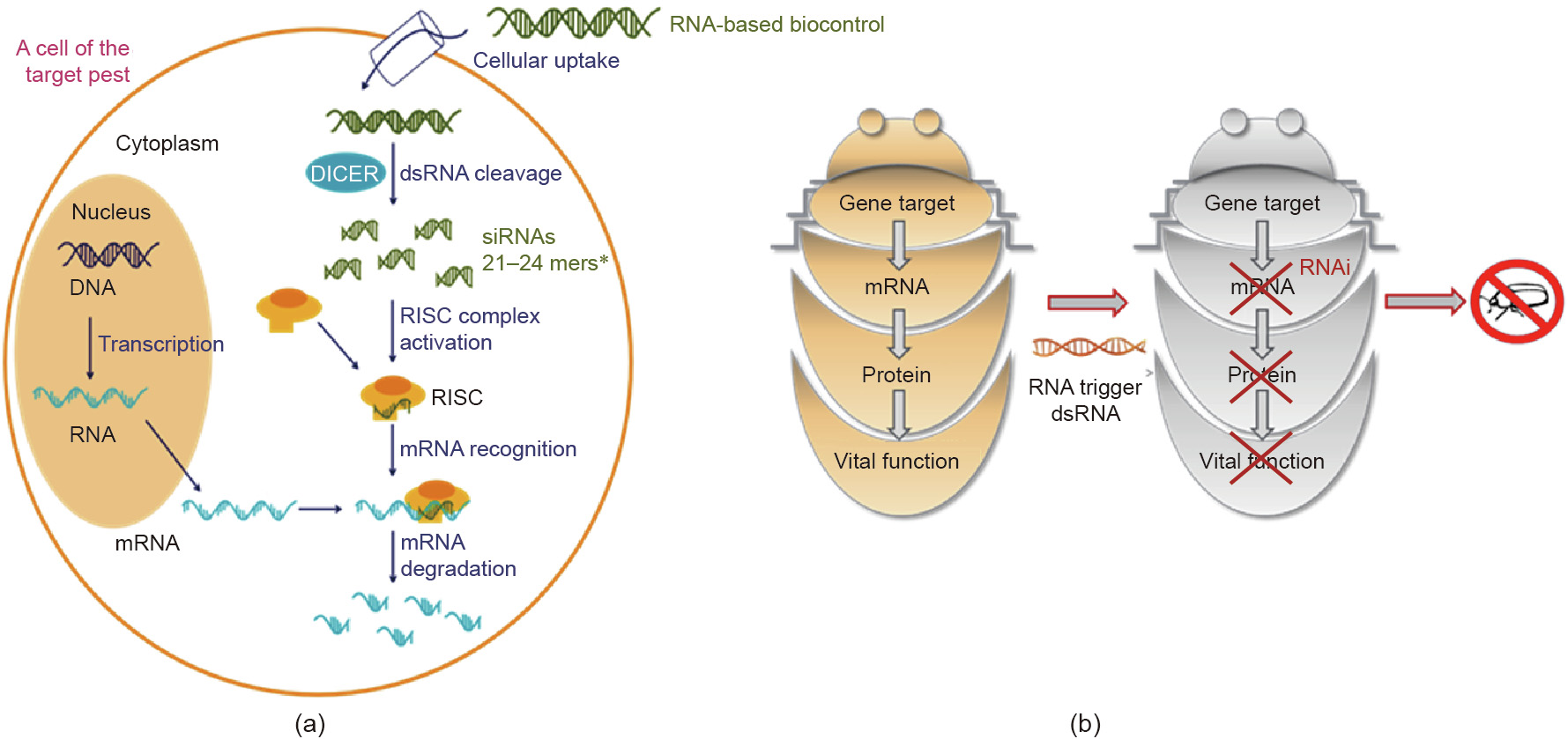
Fig. 1. (a) Molecular mechanism of RNAi; (b) principle of using RNAi for insect control. DICER: the name of a ribonuclease; RISC: RNA-induced silencing complex; siRNA: small interfering RNA.*: polynucleotides 21 to 24 bases in length.
《3. Current status of RNA-based solutions in crop protection》
3. Current status of RNA-based solutions in crop protection
The potential for the use of RNAi in agriculture has been well recognized for more than a decade [17]. Examples of early applications of RNAi include the generation of virus-resistant papayas [18] and plums [19], and the development of tomatoes with delayed ripening [20]. Today, many academic groups and agribusiness companies such as Syngenta, Bayer, Corteva, BASF, and others are actively pursuing the successful application of RNAi for the protection of crops against various insect species. While much progress has been made in understanding the potential and limitations of this approach, there are only a limited number of RNAi-related products in the marketplace. Currently, the most advanced route of application of insecticidal RNAi (with regards to commercial deployment and regulatory evaluation) utilizes in planta production of insect-specific dsRNA as a PIP, via genetic modification (GM), with one deregulated transgenic event targeting the control of corn rootworm (CRW), Diabrotica virgifera virgifera. This product, SmartStax Pro, containing a dsRNA targeting the Snf7 gene, can reduce root damage under high CRW densities and prolong Cry3Bb1 and Cry34Ab1/Cry35Ab1 durability [21], however this product is not yet available on the market. Significant competition can be expected in the area of GM applications of RNAi, with a focus on the pests of crops with large potential market sizes in order to offset the high development and regulatory costs associated with the deregulation of a transgenic product.
Fortunately, dsRNA AI can also be applied topically, and will subsequently elicit effects in sensitive insect classes [17,22]. One key requirement is the oral ingestion of the AI by the insect, as dsRNA-mediated control has not been demonstrated via a contact-only route. The spraying of dsRNA AIs using existing agronomic practices is therefore a viable route for insect control, provided the pest insect actually feeds on the treated surface. This approach is being pursued by many academic, governmental, and industrial groups [23]. The primary benefits here include the ability to respond quickly to changing pest pressure, the potential to address important yet niche markets, the usability for crops for which a GM route is not feasible or acceptable, and the potentially reduced regulatory costs.
Inherent susceptibility toward dsRNA-mediated control varies widely among common pest insect species. Control via oral ingestion has been most widely validated and characterized to date in coleopteran pests [17,24], yet tractability via topical application in a field setting does not extend to all beetle species. This variation in susceptibility can stem from multiple factors including behaviors, physical location (e.g., larvae inside the plant or in soil), and lack of appropriate uptake of the AI into the pest insect’s cells. Hemipteran pests have also been shown to be susceptible under lab and greenhouse conditions [25,26], albeit with much greater variability in observable response when compared with coleopterans [27]. Unfortunately, one of the most destructive insect orders, the Lepidoptera, has been particularly recalcitrant to control via RNA-based solutions. Although numerous publications have demonstrated that RNAi-mediated gene knock-down can occur and may, in some instances, elicit low levels of mortality [28], there is a lack of robustness and consistency in the response triggered by dsRNA. Lepidopteran control is still an exciting area of research, as potential solutions may well exist. Reports of new efforts and attempts at demonstrating control of these insects via dsRNA appear with some regularity [29,30]. Pest species with less well-characterized effects of RNAi (and commensurately smaller research efforts) include stinkbugs [31], aphids [26], and herbivorous mites [32]. Interestingly, the development of a control program to target the bee-parasitic Varroa mite using dsRNA has been reported [33].
《4. Advantages and limitations of RNA-based biocontrols in crop protection》
4. Advantages and limitations of RNA-based biocontrols in crop protection
RNA-based biocontrols offer an exciting new form of cropprotection product, and much of their potential stems from the various ways in which they differ from traditional cropprotection chemicals. As outlined above, dsRNA controls offer an entirely different mode of action than the other crop-protection products currently available. This implies suitability for use within integrated pest management (IPM) to delay or reduce emerging resistance against established chemical solutions. Similar to traditional crop-protection chemicals, the deployment of new RNAbased biocontrols will benefit greatly from such IPM systems, as biocontrols will not be immune to the possibility of resistance development [34]. Due to a mechanism that targets specific gene sequences, higher levels of selectivity are possible, completely unlike that found in traditional chemical controls. Selective control of a single pest species can even be achieved, while keeping the pest control agent inactive to species from closely related genera (as shown in Fig. 2 for the selectivity between two coleopteran pests, the Colorado potato beetle (CPB) and mustard leaf beetle (MLB)). This selectivity, as well as the uptake barriers for dsRNA that are common in many animals, could potentially lead to attractive safety profiles and a significantly reduced risk for non-target organisms (NTOs), including beneficial insects. High selectivity comes with a price, however, as it has the potential effect of reducing the inherent pest spectrum, which can limit the addressable agronomic utility.
《Fig. 2》

Fig. 2. Example of RNAi selectivity by design. Two closely related chrysomelid beetles, CPB and MLB, were treated with dsRNA fragments of a conserved target gene present in both species. Single fragments were only active in the native beetle species while a combination of CPB and MLB fragments was active in both.
Being composed of polynucleotides, RNA molecules are inherently labile, especially in harsh environments such as soil [35]. Thus, dsRNA is biodegradable; breakdown products (i.e., individual nucleotides) are present in all environments in great abundance, and have a long history of safe consumption. However, this extreme lability can be a significant hindrance when targeting the pests present in such harsh environments, as it requires substantial formulation efforts to achieve a balance of delivering sufficient activity while retaining eventual lability. In milder environments, such as on a leaf surface, testing has demonstrated that formulated materials possess enough photostability and rainfastness in the field to deliver a commercially relevant window of control.
dsRNA controls delivered as PIPs enjoy many of the same advantages and suffer from many of the same limitations as those described above for topically applied dsRNA. Some key differences stem from the mode of production. The largest difference is that a GM plant producing a dsRNA molecule will, in effect, benefit from its systemic presence—something that is unobtainable with topically applied dsRNA thus far. This means that the AI is protected from many of the degradative factors present in the crop plant’s environment, and can be protected throughout the plant’s lifecycle, without the need for spraying. However, there are accompanying challenges with this approach. The largest relative drawback is that topically applied dsRNAs can benefit from the presence of additives and co-formulants designed to protect the dsRNA from nucleases [36,37]—a key issue when addressing recalcitrant insects with highly degradative gut environments, and something that is not easily achieved through a GM application. The advantages and limitations of in planta versus ex planta application of RNAbased solutions are summarized in Table 1.
《Table 1》
Table 1 Advantages and disadvantages of in planta versus ex planta application of RNA-based solutions.

《5. Application of RNA-based biocontrols in crop protection》
5. Application of RNA-based biocontrols in crop protection
Tremendous research efforts are currently being undertaken by academic and industrial groups to identify RNA-based controls against commercial crop pest species [23]. Some recent research progress toward RNA-based biocontrols against the CPB, CRW, and soy stink bug (SSB) are exemplified in this account.
The general ex planta application principles of RNA-based biocontrols are shown in Fig. 3. The dsRNA biocontrol product is sprayed onto the plant; exposure takes place when the insect pest feeds on the biocontrol, which is then taken up into the pest’s cells. Once in the cell, the RNAi process is triggered, preventing synthesis of the essential protein in the target pest, and ultimately controlling the pest before it can cause too much damage to the crop. Once the pest is controlled, the plant matures to yield its crop. If designed for selectivity, the RNA-based biocontrol can reduce the risk to non-target insects (Fig. 3).
《Fig. 3》

Fig. 3. Application of RNA-based biocontrols in crop protection.
《5.1. Control of the CPB》
5.1. Control of the CPB
One pest that has received considerable focus is CPB (Leptinotarsa decemlineata ). This voracious pest of the potato is an ideal candidate for control via RNA-based biocontrols, as it has shown itself to be highly sensitive to—and efficient at—eliciting RNAi effects. Both the larvae and adults inhabit and damage easily treated plant surfaces, and readily consume treated leaves. These characteristics have led to efficient levels of control, measured by insect mortality and plant protection, in lab-based assays (both in vitro and on planta ). This has been taken further within Syngenta, and we have successfully translated this lab-based activity into field efficacy, as shown in Fig. 4.
《Fig. 4》

Fig. 4. 2015 field trial of a topically applied dsRNA against CPB. The potato field experienced heavy CPB pressure. Application at a commercially reasonable rate led to protection of the plants commensurate with the chemical control standard. (a) RNA-based biocontrol treated trial plot; (b) nearby untreated control plot.
NTO screening was performed with the CPB-active dsRNA that was used in these field trials, and it demonstrated excellent safety and target selectivity (Fig. 5). Datasets for this CPB-active dsRNA can be accessed at the following website: http://opendata.syngenta.agroknow.com/rna.
《Fig. 5》

Fig. 5. Example of the selectivity of the CPB dsRNA as tested on CPB versus other insect species and beneficial insects. * indicates species that have been shown in the literature to be responsive to dsRNA mediated RNA interference.
《5.2. Control of the CRW》
5.2. Control of the CRW
Syngenta is also working to extend the learnings from the CPB research program to other insect pests. While GM applications against CRW are being actively pursued (with one product now on the market), there are markets and regions (e.g., Europe) where a sprayable application approach may be more favorable. RNAbased biocontrols for soil-dwelling pests face the considerable challenge of the harsh soil environment. If they are to match the efficacy of currently marketed chemical controls, they must remain bioavailable in situ for several weeks. The biological environment of the soil, however, which contains many microbes and nucleases, leads to rapid degradation of unprotected dsRNA. Syngenta has leveraged its experience in AI formulation to improve the stability of dsRNA biocontrols in harsh environments, leading to significant improvements of the AIs stability within the soil, as shown in Fig. 6.
《Fig. 6》

Fig. 6. Bioassays in the presence of (a) unformulated and (b) formulated dsRNA targeting CRW. Each plant is infested with CRW larvae and incubated under identical conditions. The dsRNA is tested at multiple rates of application and leads to protection of the plant over the assay period. Stronger control is seen with formulated dsRNA due to protection from degradation.
《5.3. Control of the SSB》
5.3. Control of the SSB
Another research program targets the stink bug pests of soybean. These pests have demonstrated an inherent susceptibility to control via dsRNA biocontrols; however, their biology and feeding behaviors present specific challenges to be overcome. For oral uptake of the biocontrol to successfully happen, the dsRNA must survive the prolific salivary nucleases produced by the pest [38]. When this issue is taken in combination with the fact that a sucking pest, such as a stinkbug, inherently consumes less sprayed leaf surface than a chewing pest (e.g., CPB), the challenge of eliciting an RNAi effect under these conditions becomes quite clear. This is another area where Syngenta has applied formulation expertise in order to increase the stability and availability of the RNAbased biocontrol. Our efforts have resulted in the protection of soybean plants after treatment with a specific dsRNA targeting the pest stink bug species, as shown in Fig. 7.
《Fig. 7》

Fig. 7. Soybean plants treated with (a) a specific dsRNA biocontrol targeting the pest stinkbug species and (b) a negative control dsRNA sequence. These plants where then infested with adult and various larval stages of stinkbugs and incubated under identical conditions. Clear plant protection can be seen after treatment with the biocontrol.
《6. Conclusions and outlook》
6. Conclusions and outlook
Recent research has demonstrated that RNA-based biocontrols are becoming valuable new additions to the available cropprotection arsenal. They possess a unique mode of action and can be implemented via both GM and biocontrol approaches. Furthermore, they are poised to play an important role in well-designed IPM systems, which has been clearly demonstrated by the deregulation and sale of the first commercial RNA-based corn PIP. This development comes at a fortuitous time for growers, when increasing pressure from regulatory bodies and society is being exerted against existing broad-spectrum chemical controls. RNA-based AIs promise to deliver the selectivity and sustainability that are highly desirable in a future insecticide, due to their utilization of a natural process to exert control and their high level of selectivity, which results in reduced risk for NTOs. Many challenges still exist on the road toward the implementation of a broader range of RNAbased products and their widespread use and application. These challenges include a relatively slow speed to control versus traditional chemical controls and the variability in response level seen in different insect pest groups. Cost-effective manufacturing at scale remains a challenge, despite great progress in recent years. Nevertheless, it can be expected that RNA-based AIs will become valuable new tools complementing the current arsenal of cropprotection solutions.
《Acknowledgements》
Acknowledgements
The authors would like to thank Pat Bauman, Mike Bean, Andrea Burns, Sonia Herrero, Duncan Oliver, Katja Schlink, Elke Schmidt, and Jason Vincent for reviewing the manuscript and for the stimulating discussions.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Matthew Bramlett, Geert Plaetinck, and Peter Maienfisch declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




