《1. Introduction》
1. Introduction
Bioimplants are implants for medical or clinical therapy applications, such as porous bone implants, prosthetics, wearable biosensors, and drug delivery systems. They are usually implanted into the human body for a period of more than 30 days [1]. They are designed to fix, support, reproduce, or improve the functions of human tissues by integrating the human body, properties of materials, and intactness of bioimplants. Recently, the demand for bioimplants has grown tremendously due to an aging population [2] and a shortage of donor organs in medical treatment [3]. With the study and expansion of the biomaterials field [4], various bioimplants, such as brain/neural implants [5], bone/cartilage implants [6], dental implants [7], and other structural implants, have been developed. Proper selection of biomaterials, manufacturing method [8], surface treatment [9], and biological evaluations [5–8] is the major challenge with bioimplants.
According to differences in the amounts of cellular components in implants [3], bioimplants are categorized into biological implants, biologized implants, and biofunctional implants [10]. Biological implants are prepared from natural biological materials including cell proteins or others by bioprinting. They usually contain two key components: a bioprinter containing living cells and biodegradable scaffolds/matrices (hydrogels). Until now, biological implants could not be used within the human body, although bioprinting has been accepted as a robust potential technology. Biologized implants are fabricated using cellular components and biomaterials that are permanent and non-biodegradable. Bioinert materials, including stainless steel (SS; 316L), tantalum, gold, cobalt–chromium (Co–Cr) alloys, titanium (Ti), and nitinol, are widely applied as biologized implants [11]. Biofunctional implants are implants that have undergone a surface treatment by which bioactive surfaces are prepared after implantation. The surface treatments facilitate cell attachment and proliferation, which are key preconditions for tissue engineering.
Additive manufacturing (AM) technologies, which are methods that add materials onto the substrate rather than subtracting materials as in traditional manufacturing [3], provide more possibilities for the production of bioimplants with complex geometry or custom requirements with high efficiency. Recently, two inspiring papers on the 3D bioprinting of tissues or organs were published. Lee et al. [12] presented a method of printing human heart components at various scales, based on suspended hydrogels. Grigoryan et al. [13] established intravascular and multivascular architectures of an alveolar model with photopolymerizable hydrogels; this model was also used in a rodent model of chronic liver injury. At present, fused deposition modeling (FDM), selective laser melting (SLM), stereolithography (SLA), and other AM technologies [14] are being applied successfully to manufacture bioimplants, including cortical bone [15], skull [16], cartilage [17], and surgical tools [18]. However, some drawbacks exist, including additional processing, high cost, and limitations of printable materials [3]. Therefore, new printing methods and materials must be developed to overcome these limitations.
In this paper, two-dimensional (2D) and three-dimensional (3D) AM materials for bioimplants are reviewed. Furthermore, with novel ceramic inks as four-dimensional (4D) AM materials, both rigid and soft bioimplants have been developed, and the combination of soft and rigid segments within one model has been successfully achieved. These novel ceramic inks show great potential in the manufacturing of bioimplants consisting of different segments with various mechanical modules, such as knee joints and vertebral implants.
《2. 2D AM materials for bioimplants》
2. 2D AM materials for bioimplants
2D AM is regarded as a matter of applying materials to the surface of an object, typically by coating or other surface-treatment techniques. As shown in Fig. 1 [19–22], 2D AM materials are widely applied in biomedical fields. Corrosion behavior, excellent mechanical properties, and biocompatibility are vital preconditions for the performance of bioimplants in the internal fluid environment of the human body [23]. Surface chemistry and physical topography of the surface play a vital role [9]. Thus, various coating and surface-treatment methods [8], including plasma spraying, sputter coating, and ion-beam-assisted deposition, among others, have been applied to optimize the mechanical properties, biofunctionality, and biocompatibility of biomaterials such as bioceramics, bioglasses, biopolymers, and metal alloys [5,8,9,23], and to enhance the performance of bioimplants or other medical devices. Biodegradable magnesium (Mg) [24] and Ti alloys [25,26] are typical bioimplant materials due to their long-term structural and mechanical durability [24–27]. Coatings on the abovementioned metal-alloy biomaterials have been the subject of much study recently, and include a pulse-potential coating of calcium phosphate on the surface of a Mg alloy (AZ91) [28] and an ultrathin film coating of hydroxyapatite (HAp) on a magnesium–calcium (Mg–Ca) alloy [29]. Both coatings have better performance than their uncoated counterparts in a simulated internal body-fluid environment. In addition, films of Ti alloys have been fabricated onto commercial purity (CP)-Ti substrates via the magnetron sputtering method to enhance the corrosion-resistance properties [30].
《Fig. 1》

Fig. 1. 2D AM materials in biomedical applications. (a) Schematic showing diffusion of implant-derived Mg2+ contributing to osteogenic differentiation; (b) structure of supra-nanometer-sized dual-phase glass-crystal; (c) scanning electron microscopy images of corrosion regions on stents with various coatings under a simulated body environment; (d) fabrication process of microrobots including Ni/Ti deposition. DRG: dorsal root ganglion; CGRP: calcitonin gene-related peptide; TRPM7: transient receptor potential-melastatin-like 7; MAGT1: magnesium transporter subtype 1; CALCRL: calcitonin receptor-like receptor; RAMP1: receptor activity modifying protein 1; PDSC: periosteum-derived stem cell; cAMP: cathelicidin antimicrobial peptide; CREB: cAMP-regulated enhancer B. (a)–(d) Reproduced from Refs. [19–22] with permission of the authors.
Research shows that Mg in bioimplants can promote osteogenic differentiation and improve bone-fracture healing in rats [19]. Development of a supra-nanometer-sized dual-phase Mg alloy using the magnetron sputtering method was reported; this alloy exhibited near-ideal strength at ambient temperature [20]. This supra-nanometer-sized Mg alloy offers great manufacturing potential as a coating on biodegradable implants to improve wear resistance and osteogenic differentiation ability.
Natural and synthetic polymers are widely used as scaffolds or bioimplants in regeneration medicine and the field of tissue engineering [31,32]. Polymer coatings can also promote the biocompatibility and biofunctionality of bioimplants. Tremendous advances have been achieved in the polymer coating of implants. For example, polymer-coated stents can slowly release anti-stenosis pharmaceuticals [33]. Furthermore, biodegradable elastomeric polyurethanes were reported as drug-eluting coatings for degradable vascular stents based on Mg [21].
In addition to their performance on bioimplants, coatings have great potential in the fields of tissue engineering and cell therapy [34,35] as smart microrobots [36,37]. A porous microrobot fabricated by photoresist SU-8 and coated with nickel (Ni) and Ti can achieve motion control under an external magnetic field [22]. With this microrobot, targeted cells delivery in vivo can be achieved successfully under an external magnetic gradient field. A magnetically driven micro-swimmer with a Ni coating was also reported [33], and can potentially be used in medical diagnosis and treatment.
《3. 3D AM materials for bioimplants》
3. 3D AM materials for bioimplants
Although many biomaterials have been reported, not all are available for the 3D printing of bioimplants. Materials for the preparation of bioimplants should first have good biocompatibility and low toxicity. Cells should be able to adhere to the surface easily and proliferate well. As they will remain in the human body over the long term, materials should not release toxic elements, and should possess an appropriate degradation rate and wear resistance. To meet the requirements of different locations within the human body (e.g., bone [38], cartilage [39], blood vessel [40,41], joint [42,43], and other focal zones), the application environment should also be considered. Regarding the implants, a mismatch of stiffness between tissue and implants can influence the load sharing in the process of implant use. The materials for load-bearing implants (e.g., bone implants [38,44]) should have high mechanical strength. Therefore, metals and ceramics are good candidates for load-bearing implants. Polymer materials must also be considered due to their appropriate tensile strength and an elastic modulus that is similar to that of host tissues.
To fabricate implants by 3D printing methods, the chemical and physical properties of materials must also be considered. Several materials that have been used in bioimplants or have potential for bioimplant use in the near future are introduced in the following discussion.
《3.1. Polymers》
3.1. Polymers
Polymers that are commonly used to prepare scaffolds by 3D printing include polyetheretherketone (PEEK), polycaprolactone (PCL), poly(vinyl alcohol) (PVA), and poly(L-lactic acid) (PLLA) [3].
PEEK, which has been approved by the US Food and Drug Administration (FDA), is a thermoplastic biomaterial with mechanical properties that have been highlighted for use in artificial bone implants, especially at human load-bearing sites. The Young’s modulus and tensile strength of PEEK are 3.3 GPa and 110 MPa, respectively, which are in close proximity to the corresponding values of 3.75 GPa and 100 MPa for collagen [45]. Owing to its mechanical proximity with natural collagen, PEEK is a suitable candidate for replacement of collagen in artificial bone implants. Processability is a significant challenge for PEEK due to its high glass-transition temperature and melting temperature, at 143 and 343 °C [46], respectively. In cranio-maxillofacial (CMF) surgery, patient-specific implants have been fabricated by FDM [47], with collection bed and print temperatures of 100 and 400 °C, respectively. Haleem and Javaid [46] also reviewed the promising application of 3D-printed PEEK in dental implants. Zhang et al. [48] developed a new method of designing costal cartilage prostheses with wavy elastic structure by means of FDM.
PCL is the most commonly used thermoplastic polymer for the 3D printing of bone scaffolds due to its prior FDA approval, good biocompatibility, and slow biodegradation. Its low melting temperature (60 °C) eases its fabrication by means of benchtop FDM. Zamani et al. [49] prepared a PCL scaffold with gradient mechanical properties by FDM for use as potential mandibular bone implants. The PCL material lacks osteo-inductivity, so functionalized mineral additives had to be incorporated in the matrix, including tricalcium phosphates (TCPs), HAp crystals, decellularized bone matrix (DCB) [50], and trace elements (such as strontium (Sr), Mg, zinc (Zn), silver (Ag), and silicon (Si)) in the human body [51]. PCL scaffolds can also be used in the clinical application of augmentation rhinoplasty [52].
Poly(lactic acid) (PLA) is a semi-crystalline polymer with melting and glass-transition temperatures of 174 and 57 °C, respectively. PLA has two different stereoisomers, PLLA and poly-D-lactide (PLDA). PLLA degrades much slower than PLDA in the human body, so PLLA is usually made into orthopedic implants. PLA has been approved by the FDA for use as a human biomedical material, and shows promising application for fixation devices such as screws, pins, sutures, and arrows in orthopedics and dentistry due to its good biocompatibility and low toxicity. However, it presents a problem similar to that encountered with PCL: namely, its lack of mechanical strength and functionality limit its further applications [47,53].
In addition to the aforementioned polymers, poly(methyl methacrylate) (PMMA), PVA, and poly(lactic-co-glycolic acid) (PLGA) are widely used in 3D-printed implants. The usage of PMMA in cranioplasty can be dated back to the 1940s. Petersmann et al. [54] prepared cranial implants using PMMA by means of FDM. PVA shows excellent potential for cartilage repair.
《3.2. Ceramics》
3.2. Ceramics
Ceramics are preferred for the preparation of bone implants, because natural bone is composed of ceramic (HAp, ~80% wet weight of bone) and collagen. Ideal bioceramics should be not only biocompatible, but also osteoconductive and osteoinductive, without releasing toxic elements during application. Initially, bioinert bioceramics were represented by zirconia (ZrO2) [55] and alumina (Al2O3) [56] for the production of femoral heads of total hip arthroplasty prostheses. Later, bioactive ceramic materials were developed, which can form strong chemical-force bone bonding to host bone tissue without causing inflammation. Ca–P-based ceramics are a well-known group including HAp [57], TCP [58], and bioglass as bone-replacement materials. These bioactive ceramics have a significantly lower mechanical strength compared with ZrO2 and Al2O3, but show better biological response, production of proteins, and cell adhesion (osteoconduction). Chen et al. [59] reviewed the 3D printing technologies of ceramics in 2019, including slurry-, powder-, and bulk solid-based methods.
《3.3. Metals》
3.3. Metals
The 3D printing of metals or their alloys materials is usually realized by SLM or electron beam melting (EBM) [60]. Several common metals and alloys (Ti-based, SS, and Co-based) and biodegradable metals (Mg-based, Zn-based, and iron-based) for bioimplants are introduced in the following subsections.
3.3.1. Titanium-based alloys
Ti alloys have been used as biomaterials due to their good biocompatibility, low modulus, and resistance to corrosion. Ti–6Al–4V is used to fabricate clavicular implants, mandibular implants, in foot osteotomy, as flanged acetabular cups, and in other focal zones such as dental and hip implants [61]. However, Alzheimer’s, osteomalacia, and other neurological issues occur due to the release of Al and V [62–64]. Currently, Ti–6Al–7Nb [65] and Ti–5Al–2.5Fe [66] without V are fabricated and applied in femoral prosthesis stems. Surface modifications and the addition of refractory metal elements in Ti alloys are usually necessary for better wear resistance.
3.3.2. Stainless steel
Austenitic 316L SS alloys (containing 2%–3% molybdenum (Mo)) are the most commonly used implant materials to fabricate internal fixation devices (stents, bone plates, and artificial joints) [67,68]. Because 316L SS cannot promote new tissue growth, Hao et al. [69] presented a combination material comprising 316L SS and HAp bioceramic for manufacturing load-bearing and bioactive composite implants by SLM. 317L SS alloys (containing 3%–4% Mo) are better than 316L in terms of pitting and general corrosion resistance [70]. To reduce bacterial infections of bone implants, Chai et al. [70] studied the biocompatibility and antibacterial activity of 317L SS–Cu in vitro and in vivo. Localized corrosion effect, causing 24% [71] of implant failures, is a major issue impairing the performance of SS alloys as implant materials [72]. Surface modification [73], coating, and surface texture modification (at the nanoscale) [74,75] can be adopted to improve the corrosion resistance of SS.
3.3.3. Cobalt-based alloys
Co–Cr alloys, consisting of Co, Cr, Ni, and Mo, are commonly used biomaterials for orthopedic implants. Compared with SS, Co-based alloys have better biocompatibility, abrasion resistance, corrosion resistance, and mechanical strength [72]. Co–Cr alloys have two basic types: Co–Cr–Mo alloys and Co–Ni–Cr–Mo alloys. Co–Cr–Mo alloys have been used in dental implants and artificial joints, with element contents of 27%–30% Co, 2.5% Ni, and 5%–7% Mo [76]. Co–Ni–Cr–Mo alloys have been used for heavy loadbearing joints (hip and knee joints) [77], composed of 19%–21% Cr, 33%–37% Ni, and 9%–11% Mo [76]. Xiang et al. [78] manufactured a Co–Cr–Mo alloy with an anisotropy microstructure and mechanical properties by means of EBM. Biocorrosion is one of the major problems for Co-based alloys due to the release of positively charged metal ions in the process of binding to proteins or cells in the human body [79].
3.3.4. Magnesium-based alloys
Pure Mg has a similar density to natural bone, at slightly less than 1.74 g·cm-3 in comparison with 1.8–2.1 g·cm-3 [80]. However, its application for implants in the human body is restricted by its rapid corrosion, which causes quick degradation. If researchers can control the corrosion rate of Mg, it could be an appropriate candidate for bone implants, as its elastic modulus (45 GPa) is similar to that of cortical bone. This would allow the stress-shielding effect to be avoided effectively. Mg alloys (Mg–Zn- [81], Mg–Ca- [82], Mg–Si- [83], and Mg–Sr-based alloys) [84] provide good solutions to control the corrosion of pure Mg, and have shown great potential in the tissue engineering, orthopedic, and cardiovascular fields. In bioimplants, Al and rare-earth elements cannot be alloyed with Mg, because these two components result in neurotoxicity and hepatotoxicity, respectively, although the mechanical strength is increased. In addition, hydrogen formation is usually a common challenge in research on Mg alloys [5,85]. As an alternative material, Mg glasses can be applied as implants without the formation of hydrogen [86].
3.3.5. Zinc-based alloys
Zn-based alloys hold tremendous potential as tissue implant materials due to their good biodegradability and biocompatibility [87]. In 2013, Bowen et al. [88] reported Zn as a superior applicant material for the fabrication of stents. Pure Zn has an ultimate tensile strength of 20 MPa by casting and 120 MPa by wrought operation [89,90]. However, it is not strong enough for application in stents (i.e., 300 MPa is required for a vascular stent). Thus, Zn needs to be combined with other metals to form alloys for biomedical applications. Zn–Mg, Zn–Ca/Sr, Zn–Al, Zn–Li, Zn–Ag, Zn–Cu, and Zn–Mn alloy systems have been reviewed in some papers [87,91]. Zn–Cu alloys show potential application in craniomaxillofacial osteosynthesis implants [92]. Interfacial Zn–P provides a key controlling biocompatibility for Zn implants, and can be used as a promising coating material with stable chemical properties for other biomedical applications [93].
3.3.6. Iron-based alloys
Iron and its alloys, which do not promote hydrogen evolution and which have better mechanical properties than Mg-based alloys, are another type of biodegradable metal that can be used as cardiovascular stent or bone implants [94] due to their low hemolysis ratio and excellent anticoagulant property [95,96]. The elastic modulus of pure Fe (211.4 GPa) is higher than that of pure Mg (41 GPa) or 316L SS (190 GPa) [97]. Mn, carbon (C), Si, and palladium (Pd) elements are commonly alloyed with Fe in order to enhance the degradation rate of pure Fe material (0.16 mm·a-1 [97] in an osteogenic environment) and reduce its magnetic susceptibility in clinical application. When an open cell structure is designed for Fe-based alloys, they can show mechanical properties close to those of natural bone [98]. Li et al. [99] fabricated Fe scaffolds by direct metal printing with an ordered porous structure, and studied their biodegradation behaviors and mechanical properties. Hong et al. [100] fabricated Fe–Mn and Fe–Mn–Ca-based constructs by 3D printing, and found that Ca addition could enhance the degradation rate of Fe. Usually, modification needs to be done to improve the surface bioactivity by stimulating bone formation. Yang et al. [101] presented a HAp coating method based on a hydrothermal procedure to improve 3D-printed Fe scaffolds, and demonstrated potential application in bone engineering.
3.3.7. Bulk metallic glasses
Bulk metallic glasses (BMGs) are a kind of metallic material with a strength, elasticity, and Young’s modulus of ~2 GPa, ~2%, and 50–100 GPa, respectively [102]. They have high wear- and corrosion-resistance ability due to their unique disordering atomic structure. Therefore, BMGs can be used as novel implant materials with better biocompatibility than their crystalline bulk. Zr44Ti11Cu10Ni10Be25 BMG exhibits better cell growth and attachment support ability [103]. Pt57.5Cu14.7Ni5.3P22.5 BMG with micro-/nano-patterns surface shows enhanced angiogenic performance [104].
《3.4. 3D AM technologies for bioimplants》
3.4. 3D AM technologies for bioimplants
Several representative 3D AM technologies that are commonly used for bioimplant manufacturing are summarized in Table 1 [14–17,105–113], along with their advantages, limitations, and current applications.
《Table 1》
Table 1 Several representative 3D AM technologies for bioimplant manufacturing.

3.4.1. Fused deposition modeling
FDM is an AM process in which a thin filament of polymer is formed after melting and extrusion [114]. Thermoplastic polymers are usually used, such as polycarbonate (PC), acrylonitrile butadiene styrene (ABS), PCL, polyamide (PA), and PLA. Zeng et al. [115] built an artificial human ear using FDM technology, in which polyurethane was adopted to prepare the auricular framework, due to its good flexibility and biocompatibility. This experiment indicated an efficient way to achieve macrotia reconstruction and mitigate other cartilage defects in orthopedic surgery. Gronet et al. [116] fabricated acrylic cranial implants for two patients that facilitated the restoration of large or complicated cranial defects. Tan et al. [16] produced patient-specific acrylic cranioplasty implants. The advantages of these implants included no required chemical post-treatment and cost efficiency, while their main limitation was the inferiority of the mechanical properties of the products [117].
3.4.2. Direct ink writing
Direct ink writing (DIW) is an AM process in which the suspension or melts of the material are extruded from the machine as filaments [105]. There are abundant sources of materials for DIW, including hydrogels, ceramic/metal powder suspensions in polymer solution, and thermoplastic polymer melts. Recently, a meltelectrospinning-based DIW method [118] was developed that allowed high resolution to be obtained due to the melt’s stretching under a high-voltage electric field. Similar to FDM, the printing resolution is subject to, for example, the pressure, moving velocity of the stage, and diameter of printing nozzles. For ceramic and metal parts, post-treatment is required to remove the polymer composition in the printing inks by sintering or other methods. The DIW method has been applied in developing bioimplants, including in cartilage [17], bone tissue [106], and vascularization [107].
3.4.3. Stereolithography
SLA, one of the earliest AM methods, was developed in 1986 [119]. It uses ultraviolet (UV) light to initiate polymerization on a thin layer of photosensitive resin or monomer solution. An acrylic or epoxy group is usually contained in the monomers, which can be activated to polymerize and form long-chain polymers. Bone [15] and dental implants [108] have been developed using the SLA method. To prepare ceramic-based implants, ceramic powders can be dispersed in the photosensitive resin, and the polymers are removed by subsequent sintering. Winder and Bibb [120] summarized the potential applications of SLA in maxillofacial prosthodontics, including auricular and nasal prosthesis, obturators, and surgical tents. Post-treatments sometimes include, for example, polishing, painting, and sanding. The advantage of SLA is the production of implants with a high resolution of 10 μm [119], while its disadvantages are a high cost and limited material resources.
3.4.4. Selective laser melting
During SLM processes, the laser fuses the powder, which lies loosely in the bed, at a specific location for each layer to form designed structures. Pure metal powders and laser beams (generally neodymium-doped yttrium aluminum garnet, 1.064 μm) with a higher absorptivity to metals or fiber lasers (1.09 μm) with a shorter wavelength are commonly used in the SLM process. Researchers produced the first patient-specific Ti–6Al–4V jaw implant using SLM technology [121]. The advantages of SLM include a wide range of materials to use and the recyclability of unused particles. Its disadvantages are that the printing accuracy depends on particle size and the printing environment is inert gas, preventing the oxidation of particles. Applications of SLM in bioimplants include the development of locking plates [18], acetabular cups [109], spinal surgery templates [110], and dental alloys [111].
3.4.5. Electron beam melting
EBM, which was developed by Arcam AB Corporation (Sweden) in 1997, is a powder-bed-based AM technology [112]. It is similar to the SLM process. However, an electron beam is the heat source in EBM instead of a laser beam. Moreover, EBM is only applied for the preparation of metals and alloys, while SLM is used for polymers, metals, and ceramics. EBM can operate at extremely high velocities (up to 105 m·s-1 ) under vacuum conditions [112]. Co–Cr–Mo alloys [78], Ti–6Al–4V [113,122,123], and Ni–Ti alloys [124] have been reported to be fabricated by EBM as orthopedic and dental implants. A noteworthy advantage of EBM is that the level of residual stresses in the parts made by EBM is much lower than that in those made by SLM.
3.4.6. 3D bioprinting
Unlike other 3D printing technologies, 3D bioprinting uses bioinks as printing materials. Bioinks consist of biological materials, biochemicals, and living cells. The central challenge in 3D bioprinting is recapitulating biological function based on the production of structures [125]. Based on the work principles, 3D bioprinting methods can be classified as inkjet-based [126], microextrusion-based [127], and laser-assisted bioprinting [128]. An ideal bioink is important for bioprinting; it should meet requirements such as an adequate mechanical strength, adjustable gelation process, biocompatibility, and so on. Researchers can refer to some reviews [129–132] on bioinks for 3D bioprinting. Kang et al. [133] invented an integrated tissue–organ printer system, which was used for the potential reconstruction of mandible bone, calvarial bone, ear cartilage, and skeletal muscle. Lee et al. [12] presented a new 3D bioprinting method with freeform reversible embedding of suspended hydrogels as a supporting printing medium, and printed a human heart at various scales. Grigoryan et al. [13] established intravascular and multivascular structures with photopolymerizable hydrogels by means of SLA. 3D bioprinting technology has shown a flourishing foreground, although it has not been used for real application in clinics.
《4. 4D AM materials for bioimplants》
4. 4D AM materials for bioimplants
In 4D printing, a 3D-printed material autonomously and programmably changes its configuration or function in response to environmental stimuli, such as stress, light, liquids, temperature changes, magnetic fields, gas pressure, embedded circuitry, or a combination of these stimuli. The first demonstration of 4D printing was a multi-material strand folded into the letter "MIT” in 2014 [134]. Thus far, many materials, including polymers [135–143], metals [143–145], and ceramics [146], have been developed for 4D AM. The shape-morphing capabilities of 4D AM materials [147] can be applied in dynamic and versatile human environments, such as for drug delivery [148] and stent insertion [149]. However, the response speed and mechanical robustness of shape-morphing materials are usually critical limitations in developing practical applications.
Gladman et al. [135] reported a biomimetic 4D printing system made of hydrogel composite materials, in which the orientation of embedded non-swelling cellulose fibers was precisely patterned for anisotropic swelling behavior. Complex-shaped overall architectures with mixed Gaussian curvature were designed and achieved with this system. Another kind of widely used 4D AM polymer is shape memory polymers (SMPs) and their composites, which have many advantages, including large deformation, multi-stimuli response, biocompatibility, light weight, and low cost [150]. Ge et al. [136] developed printed active composite materials with multi-material printing technology. The printed SMP fibers in an elastomer matrix could drive the shapemorphing behavior of the composite materials, resulting in the thermo-mechanical programming of origami patterns [137]. Ding et al. [138] designed a direct 4D printing method that integrated the programming steps into the 3D printing process, resulting in permanent programmed shapes. Lin et al. [139] introduced magnetic powders into an SMP matrix to achieve remote control of 4D-printed biomedical devices. Huang et al. [140] proposed a kind of novel 4D AM polymer made from hydrogel and SMPs; the ultrafast digital printing that was used for this polymer overcame the limitations in AM speed by avoiding layer-by-layer printing in the vertical dimension and line-by-line printing in the planar dimension. Furthermore, some works on the 3D printing of shape memory alloys (SMAs) suggest potential for developing 4D AM metallic materials [143], including Ni–Mn–Ga SMAs using the binder jetting printing method [144] and NiTi alloys using SLM methods [145]. In these works, 3D-printed SMAs exhibit shape memory behavior resulting from martensitic transformation with temperature changes.
4D AM techniques are developed with the shape-morphing capability of the relevant 4D AM materials. Various materials and technologies provide tremendous possibilities for designing and fabricating 4D smart structural materials such as soft robots, controlled grippers, programmable shape change patterns, and more. Heat is the most common and readily accessible stimulus for 4D printing, and a large number of heat-responsive 4D materials have been reported, including hydrogels [135], SMAs [151], and SMPs [138]. However, the relatively slow response speed of heatdriven 4D printing technologies is a major limitation in their broad application.
Light-driven 4D technology has attracted a great deal of attention due to advantages such as a fast response, wireless control, accurate focusing, and sustainable properties [152–154]. Graphene and carbon nanotube-based composites [155–157], liquid crystal elastomer-based composites [158–160], SMPs [161], and hydrogels [162] are commonly used in light-triggered 4D systems. A lightsensitive printed micro-swimmer [163] was reported. Humidity has also been used to drive the deformation of 4D printed actuators. Mao et al. [142] demonstrated a hydrophilic/hydrophobic bilayer under a humidity stimulus, which showed potential application in soft actuators. Magnetic fields are another important strategy for 4D material development, as their delicate control and excellent biocompatibility for living organisms meet the preconditions for their biomedical and therapy use. 4D magnetic butterfly structures [164,165] and a 4D flower-like magnetic actuator [166] were printed by DIW. Various biomimetic 4D structures controlled by a magnetic field were also reported, including spirulina cells [167], caterpillars [168,169], starfish [170], and jelly fish [171]. Besides these technologies, stress- [146], electricity- [172], and gas-driven [173] 4D printing technologies have also been studied. These 4D-driven technologies have progressed a great deal recently; however, most existing smart 4D structures are only responsive to one stimulus, which limits their interaction capacity with the surroundings and their adaptability under multiple environmental stimuli [174]. The development of multi-responsive materials and technologies has promoted a new generation of 4D-printed structures, including light-thermal dual-responsive hydrogels, electrothermal and electrochemical actuation materials [175], magnetic-photo/thermal dual stimuli actuators [176–180], temperature-pH sensitive fluorescence bilayer actuators [181], and humidity–temperature–light triple-responsive hydrogels [174]. A biomimetic shape–color double-responsive 4D composite based on SMPs and thermochromic pigments was printed by means of FDM [182]. Meanwhile, some problems for 4D AM techniques still remain to be overcome; for example, the wavelength limitation and biological toxicity of light, reactive nature concerns, and frequency control of a magnetic field.
The first ceramic 4D printing system was previously developed by our group. In this system, elastic ceramic precursors were printed, deformed, and then transformed into rigid ceramic structures, as shown in Fig. 2 [146]. The shape-morphing process can be achieved by releasing the elastic energy stored in the pre-strained ceramic precursors, which can be stretched to over 200% strain. Moreover, strength-scalability synergy is achieved in 4D printed elastomer-derived ceramics (EDCs). Hierarchical EDCs with printed architectures from 200 μm to tens of centimeters, as well as a compressive strength of 547 MPa at 1.6 g·cm-3 , can be prepared by this method [146].
《Fig. 2》
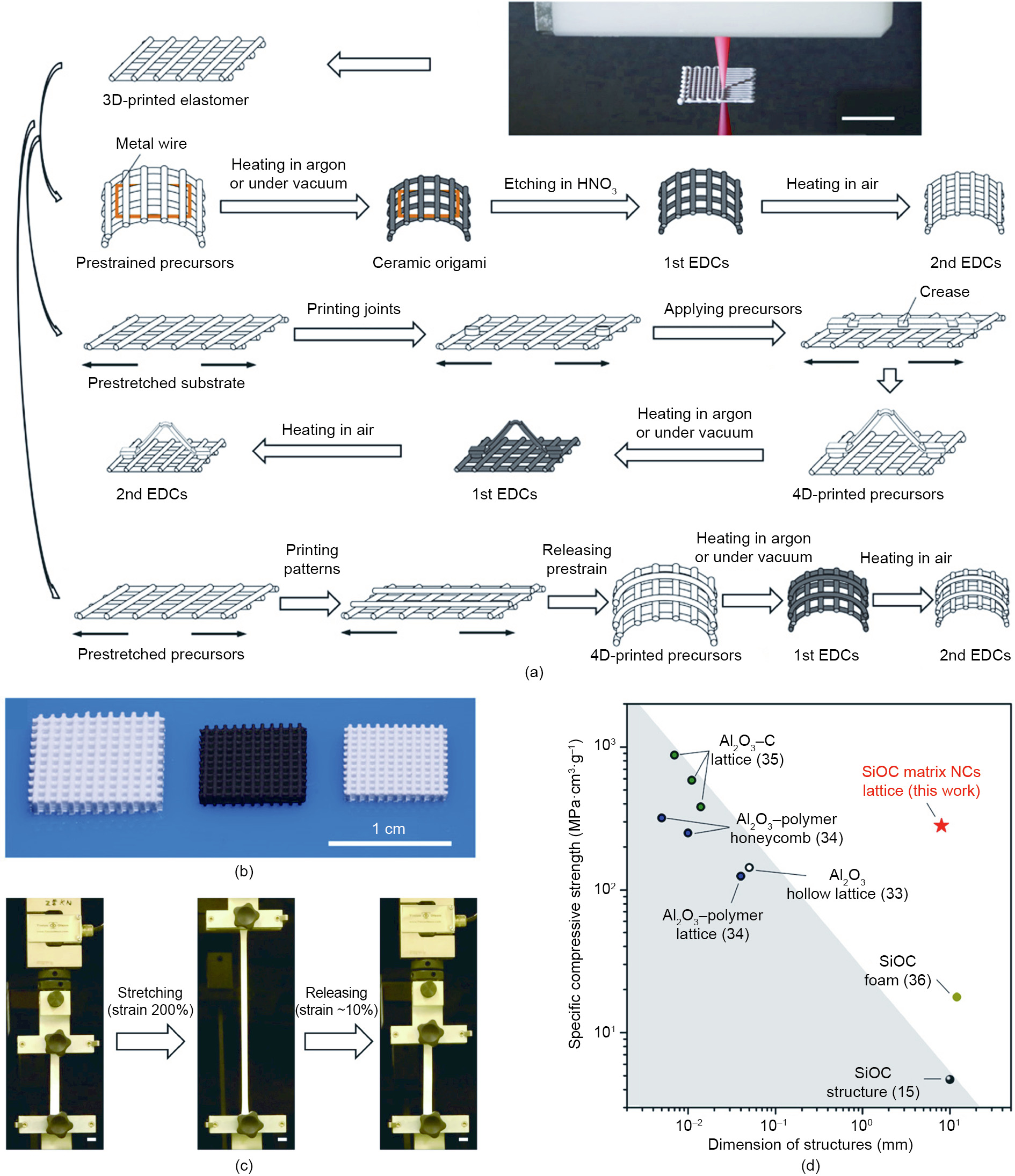
Fig. 2. A ceramic 4D printing system. (a) DIW-morphing-heat treatment method. (b) Printed micro-lattice of ceramic precursors and two types of corresponding EDCs (left to right). (c) Printed ceramic precursors can be stretched to 200% strain. (d) Strength–scalability synergy is achieved. Scale bars, 1 cm. NCs: nanocomposites. Reproduced from Ref. [146] with permission of the authors.
Compared with other 4D AM polymeric and metallic materials, the abovementioned ceramic 4D AM system has advantages for high-temperature structural applications, since polymer-derived ceramics exhibit remarkable thermal, chemical, and mechanical stability [183]. The DIW and heat treatment process of the ceramic precursors is more cost-effective than other high-melting-point material 4D AM systems, because it does not require expensive laser energy for the 3D printing process, or sintering of highmelting-point material powders for post-processing. Moreover, the ceramic 4D AM system can be extended to develop various material and shape-morphing systems, due to its open-end feedstock system for ink materials. The disadvantages of the current ceramic 4D AM system include its deformation control accuracy, because the shape-morphing system relies on a homemade biaxial stretch device. With the involvement of a new deformation mechanism and high-accuracy machining technologies, improvement is expected in the shape-morphing programming accuracy.
The above-described ceramic 4D printing work could drive innovation in the AM of bioimplants. With the printability of both ceramics and ceramic precursors, the AM of rigid bioimplants with ceramics, including crowns, locking plates, acetabular cups, and bone plates (as shown in Figs. 3(a)–(d)), as well as soft ones with ceramic precursors, including ears, tracheas, meniscuses, and ligaments (as shown in Figs. 3(e)–(h)), can be anticipated. Moreover, bioimplants with simultaneously soft and rigid parts can be printed, since the ‘‘ceramic inks” used for printing ceramic precursors and ceramics can fuse these parts together, resulting in a homologous sandwich structure (as shown in Fig. 3(i)). The inks for these results were prepared by mixing liquid PDMS (SE1700 clear, Dow Corning Co., USA) with 10 wt% ZrO2 nanoparticles (Tong Li Tech Co. Ltd., China). The 3D printing of the ceramic precursors was conducted with a DIW-based 3D printer. After ink deposition, the formed structures were post-cured at 150 °C for 30 min. Ceramics were generated by heating the ceramic precursors to 1300 °C for 1 h under argon (Ar) flow.
《Fig. 3》
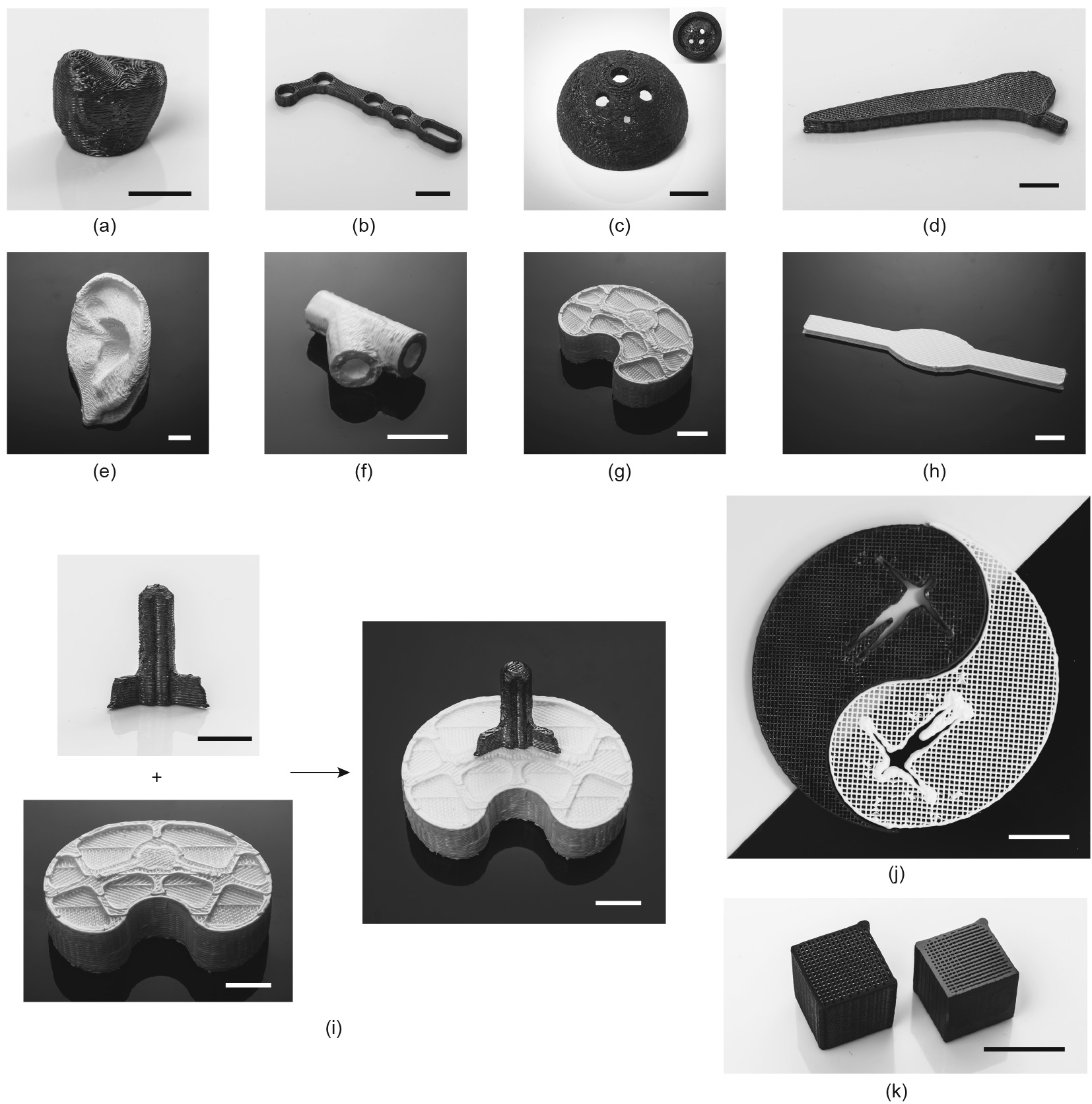
Fig. 3. Development of representative bioimplants with 4D printing hybrid ceramic precursor/ceramic materials. Printed rigid ceramic-made (a) crown, (b) locking plate, (c) acetabular cup, and (d) bone plate. Printed soft ceramic precursor-made (e) external ear, (f) trachea, (g) meniscus, and (h) ligaments. (i) Printable "ceramic inks” as adhesives to fuse printed rigid and soft bioimplants together. (j) Printed Yin/Yang symbol for Tai Chi with white/black hybrid ceramic precursor/ceramic materials demonstrating soft/rigid hybrid biological structures in the human body. (k) Comparison of printed ceramic lattice structure without (left) or with (right) polishing. Scale bars, 1 cm.
Furthermore, these printed ceramics can achieve a compressive strength of 34–547 MPa and a tensile strain of 200% [146]. According to a related report, the ultimate tensile strains for tendons/ ligaments and articular cartilage are 10%–15% and 60%–120%[184], respectively, while the compressive strengths of cortical bone tissue and dental tissue are 88–164 and 295 MPa, respectively [185]. All these parameters are within the mechanical properties range of this material, which indicates its potential use in bioimplants of various tissues, especially parts with both soft and rigid segments.
Similar to the philosophical relationship between the two fundamental elements Yin and Yang in Tai Chi, soft and rigid biological structures in the human body fuse and function together, resulting in a comprehensive balance in the form of soft/rigid hybrid biological components such as knee joints and vertebrae (Fig. 4). A Yin/Yang symbol for Tai Chi with white/black hybrid ceramic precursor/ceramic materials demonstrating soft/rigid hybrid biological structures in the human body was printed (Fig. 3(j)). Soft/rigid hybrid structural materials based on AM techniques will be desirable for a diversity of fields, including bio-inspired tough or otherwise superior materials [186–189], as well as actuators [190–192].
Post-treatment of printed structures can be applied to obtain good mechanical properties or biocompatibility. For example, post-treatment with surface mechanical attrition treatment [193] of 3D-printed components in Ti alloys can introduce a nanostructured layer to the metal surface [194] and drastically enhance fatigue resistance [195]. Furthermore, printed ceramic structures with good polishing can be prepared with an average surface roughness (Ra) on the polished samples of 0.06 lm (Fig. 3(k)).
《Fig. 4》

Fig. 4. Soft and rigid biological structures in the human body fuse and function together, resulting in a comprehensive balance like the Yin and Yang symbols in Tai Chi.
《5. Conclusions》
5. Conclusions
The present soft/rigid hybrid 4D AM concept that is achieved by the 4D printing of hybrid ceramic precursor/ceramic materials can be extended to other binary and multiple-component systems. Assisted by other technologies, such as multi-material printing and local ceramization, more kinds of soft/rigid hybrid structures with functional gradient interfaces can be additively manufactured. More innovations in the development of bioimplants for complex and dynamic biological environments in the human body could be generated together with a combination of 2D/3D/4D AM materials.
In future research, multi-material printing would include material combinations among not only printing host materials but also printing support materials and printing medium materials. With the development of multi-modulus ink material systems, AM of bio-inspired tough hybrid systems could be applied in structural materials to overcome the strength-toughness tradeoff. Multidimensional AM will drive the printing dimension to increase from 2D/3D/4D to even higher dimensions and the printing strategy to change from dot-by-dot/line-by-line/sheet-by-sheet/volume-byvolume AM to AM with even higher dimensional elements, resulting in a high level of structural freedom and printing efficiency. Furthermore, the involvement of other manufacturing strategies such as subtractive manufacturing, together with the preprogramming, real-time treatment, or post-processing of printed materials for various functional applications, will offer promising research and industrial opportunities for future study on AM materials and technologies.
《Acknowledgements》
Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFA0204403), the Major Program of the National Natural Science Foundation of China (51590892), the General Research Fund Research Grants Council (Hong Kong) (CityU 11209918), the Hong Kong Collaborative Research Fund Scheme (C4026-17W), the Hong Kong Theme-based Research Scheme (T13-402/17-N), and the Shenzhen–Hong Kong cooperation zone for technology and innovation (HZQB-KCZYB-2020030).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Guo Liu, Yunhu He, Pengchao Liu, Zhou Chen, Xuliang Chen, Lei Wan, Ying Li, and Jian Lu declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




