《1. Introduction》
1. Introduction
Aromatic aldehydes such as benzaldehydes are essential and highly desirable fine chemicals due to their wide applicability [1]. Industrially, benzaldehyde can be obtained by the chlorination of toluene and subsequent saponification [2]. This, synthetic process generally employs the use of potent oxidants like CrVI, ClO–, Br2, or peroxy acids, leading to the over-oxidation of target product and thereby its poor selectivity. Nowadays, with the increasingly prominent environmental and energy problems, and the continuous requests for improvement of industrial processes with energy-extensive consumption, laborious post-treatment and difficult separation, such as benzaldehyde production, there is an urgent need to develop environmentally-benign, synthetic processes.
Recently, photocatalysis, which is considered a promising and green strategy, has drawn the attention of researchers towards driving chemical reactions to produce valuable compounds [3,4]. Compared with traditional heating treatment, the utilisation of solar power can be a potential energy-source for industrial production processes, as it allows for energy conservation and environmental protection. Therefore, based on the principles of green and sustainable chemistry, the selective conversion process of benzyl alcohol to produce benzaldehyde using semiconductors, can be considered as one of the most promising ways. So far, multifarious semiconductors (i.e., metal oxides, nitrides, sulfides, etc.) have been used for the oxidation reaction of benzyl alcohol [1,4–8]. Unfortunately, most of them suffer from high production cost, environmentally hostile production processes, and poor selectivity. As such, it is highly challenging to design a novel and economical photocatalyst for efficient synthesis of benzaldehyde in aqueous phase. Graphitic carbon nitride (g-C3N4) has excellent characteristics, such as stable physicochemical properties, nontoxicity, and a suitable band gap (2.7 eV) for the absorption of ultraviolet (UV)– visible light. Moreover, it can be obtained from various abundant and inexpensive feedstocks, such as cyanamide, urea, melamine, and thiourea. However, significant decrease of photocatalytic activity is usually reported for g-C3N4 due to the rapid recombination of photogenerated electrons and holes (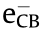 and
and  ), which significantly decreases the photocatalytic efficiency. The aforementioned drawback can be effectively settled by coupling with various semiconductors [9].
), which significantly decreases the photocatalytic efficiency. The aforementioned drawback can be effectively settled by coupling with various semiconductors [9].
Polyoxometalates (POMs) are composite of transition metal oxide clusters, which are a huge and rapidly expanding family. Due to POMs’ unique chemical structures and numerous characteristics in accordance with semiconductor metal oxide clusters, they are generally regarded as the analogs of the latter and exhibit excellent photochemical performance in various chemical reactions [5]. Upon irradiation with light energy, the surface of photocatalyst can trap the photogenerated electrons and holes, and thereby the reactive oxygen species of  or/and ·OH radicals are formed to facilitate the photocatalytic reaction. Since the photocatalytic oxidation reaction of benzyl alcohol under solar light irradiation was reported using a POMs catalyst ([S2W18O62] 4–) [10], the unique charms of POMs in photocatalytic synthesis of chemicals with high added value, especially aromatic aldehydes, were discovered [5,11].
or/and ·OH radicals are formed to facilitate the photocatalytic reaction. Since the photocatalytic oxidation reaction of benzyl alcohol under solar light irradiation was reported using a POMs catalyst ([S2W18O62] 4–) [10], the unique charms of POMs in photocatalytic synthesis of chemicals with high added value, especially aromatic aldehydes, were discovered [5,11].
Herein, we report for the first time the fabrication of a heterogeneous photocatalyst (PW12-P-UCNS; where PW12 is H3PW12O40·xH2O and P-UCNS is phosphoric acid-modified unstack g-C3N4) by immobilising phosphotungstic acid onto a g-C3N4 surface modified with phosphoric acid. This photocatalyst is applied in a green photocatalytic reaction system, for selective photo-oxidation to produce benzaldehyde in aqueous phase. The as-prepared PW12-P-UCNS exerted outstanding photocatalytic oxidation performance, attributed to three factors. Firstly, the incorporated POMs acted as superior electron acceptors to curb the recombination of photogenerated electrons and holes. Secondly, the P-UCNS with phosphoric acid-modified surface improved the absorption of O2 to generate more active species ( radicals). Thirdly, the interfacial photogenerated electrons within PW12-P-UCNS followed a Z-scheme mechanism, which obtained an efficient charge separation by the fast transfer pathway. Moreover, the high oxidation activity and reusability of the PW12-P-UCNS photocatalyst revealed a broad and promising application prospects.
radicals). Thirdly, the interfacial photogenerated electrons within PW12-P-UCNS followed a Z-scheme mechanism, which obtained an efficient charge separation by the fast transfer pathway. Moreover, the high oxidation activity and reusability of the PW12-P-UCNS photocatalyst revealed a broad and promising application prospects.
《2. Experimental section》
2. Experimental section
《2.1. Chemicals and reagents》
2.1. Chemicals and reagents
All solvents and chemicals were provided from Energy Chemical (China), were of analytical grade, and were used without further treatment.
《2.2. Fabrication of UCNS》
2.2. Fabrication of UCNS
Bulk g-C3N4 was synthesised according to Ref. [12]. A quantity of 0.9 g of bulk g-C3N4 was dispersed into 150 mL HCl solution (14.8 wt%) followed by sonicating for 1 h and stirring for another 24 h. Subsequently, it was subjected hydrothermal treatment at 110 °C. After 5 h, the suspension was centrifuged, filtrated, washed with water, and finally dried at 80 °C overnight, leading to the formation of unstack g-C3N4 nanosheets (UCNS).
《2.3. Fabrication of PW12-P-UCNS》
2.3. Fabrication of PW12-P-UCNS
As shown in Fig. 1, 0.1 g UCNS was scattered in 100 mL of 0.3 mol·L-1 H3PO4 solution and the above was stirred for 5 h to allow for adsorption of phosphoric acid on the surface of UCNS. The P-UCNS was collected by centrifugation. After heating at 60 °C in an oven for 180 min, it underwent heat-treatment at 300 °C for 90 min. P-UCNS was washed using distilled water and then dried, in order to remove weakly bound phosphoric acid anions from its surface [13]. Subsequently, predetermined quantity of H3PW12O40 (PW12) was placed in a 20 mL P-UCNS suspension of anhydrous ethanol, followed by stirring. After drying overnight, the PW12-P-UCNS photocatalyst was finally obtained.
《Fig. 1》

Fig. 1. Illustrated representation of the synthetic process for the PW12-P-UCNS.
《2.4. Characterisation》
2.4. Characterisation
Transmission electron microscopy (TEM) was performed using a HT7700 machine. A Shimadzu XRD-6000 diffractometer was used to collect the X-ray diffraction (XRD) patterns within the range of 3°–70°. A Bruker Vector 22 infrared spectrometer was employed to collect Fourier transform infrared spectroscopy (FTIR) spectra within the range of 400–4000 cm-1 . Thermogravimetric analysis (TGA) was conducted using a TGA/DSC 1 machine with small furnace (SF; temperature range to 1100 °C) from METTLER TOLEDO, USA, under nitrogen atmosphere. X-ray photoelectron spectroscopy (XPS) was conducted using a Quantera SXM machine from ULVAC-PHI Inc., Japan. High-resolution transmission electron microscopy (HRTEM) was carried out using a JEM-2010 electron microscope (JEOL Ltd., Japan). Porosimetry analysis was conducted using a ASAP 2020M machine (Micromeritics Instrument Corporation, USA).
《2.5. Performance tests》
2.5. Performance tests
The photocatalytic performance of as-prepared PW12-P-UCNS was systematically investigated using benzyl alcohol photooxidation as a model reaction to synthesis benzaldehyde under Xe light (300 W) at room temperature. Firstly, 20 mg of the prepared catalyst was scattered into 10 mL reactants solution (10 mmol·L-1 ). Before light illumination, the reactive system was placed in the dark for 30 min with continuous stirring, in order to reach the adsorption–desorption equilibrium between reactants and the catalyst. Subsequently, oxygen was bubbled into the mixture and sustained for 2 h. After irradiation, an 1 mL mixture was taken out, followed by centrifugation and filtration to separate the photocatalyst. Gas chromatography was employed using GC-2010 Pro (with HP-5 chromatographic column: inner diameter = 0.25 mm, length = 30 m; Shimadzu Corporation, Japan), to analyse and identify the products, using cyclooctane as an internal standard, and thus calculate the benzyl alcohol conversion and benzaldehyde selectivity.
《2.6. Photoelectrochemical tests》
2.6. Photoelectrochemical tests
Experiments were conducted on a typical CHI 760E electrochemical workstation (CH Instruments, Inc., USA), using a 300 W Xe lamp as a light source. Photocurrent analysis was conducted in Na2SO4 solution (0.1 mol·L-1 ) by selecting Ag/AgCl and Pt wire as the reference and counter electrode, respectively. Working electrodes were prepared as follows: 5 mg catalyst was suspended in 1 mL of ethanol solution using ultrasonication. A volume of 80 μL of the above slurry was coated on an ITO substrate and left to dry. The cyclic voltammogram (CV) plot and Mott-Schottky measurements were conducted as shown in previous work [1,12].
《3. Results and discussion》
3. Results and discussion
《3.1. Synthesis of catalysts》
3.1. Synthesis of catalysts
As shown in Fig. 1, the PW12-P-UCNS was successfully fabricated using hydrothermal treatment and an immersion process. Briefly, bulk g-C3N4 was obtained by firstly treating it with urea at 550 °C. Then, g-C3N4 was exfoliated via a HCl-assisted hydrothermal treatment and sonication. The above powder was further modified by phosphoric acid and PW12-P-UCNS was finally prepared after the incorporation of PW12. During the preparation of the PW12-P-UCNS, the –NH2 groups from the edges of P-UCNS were protonated by the acid to form [–NH3+][H2PW12O40–] species at the interface between PW12 and P-UCNS. The leakage of the Keggin units can be avoided due to the strong bonding of PW12 and P-UCNS via acid-base and electrostatic interaction. Moreover, the incorporation of PW12 accelerates the transfer and separation of charge carriers.
《3.2. Compositional and structural information》
3.2. Compositional and structural information
As shown in Fig. 2, the XRD patterns can be used to characterise the chemical structure of bulk g-C3N4, UCNS, P-UCNS, PW12, and PW12-P-UCNS. The two broad peaks positioned at 27.5° and 13.012, on the bulk g-C3N4 spectra are matched to (002) and (100) of the graphitic carbon materials, respectively [12,14]. The slight shift of (002) peak for the UCNS and P-UCNS compared with bulk g-C3N4 is attributed to the protonation of heterocyclic N atoms (C–N=C) [15–17]. Moreover, it can be seen that the shifts of PUCNS in higher 2θ angles are due to the lower extent of stacking between nanosheets [18,19]. For as-prepared PW12-P-UCNS, it shows that the characteristic peaks of the Keggin structure (2θ of 8°–11° and 18°–30°) and P-UCNS (at 2θ of 14° and 28.2°) in the XRD diagram, indicating that the structures of Keggin units and P-UCNS supports are remained after the incorporation of PW12.
Further, structural information of as-prepared PW12-P-UCNS was obtained through FT-IR measurement. Typically, g-C3N4 shows peaks centered at 890 and 810 cm-1 , attributed to the breathing mode of the heptazine ring (Fig. 2(b)) [20]. The signals centered at 1637, 1570, and 1463 cm-1 can be attributed to the  , while the peaks observed at 1416 cm-1 can be assigned to
, while the peaks observed at 1416 cm-1 can be assigned to  [21,22]. Moreover, the signals appearing at 1248 and 1327 cm-1 can be assigned to the
[21,22]. Moreover, the signals appearing at 1248 and 1327 cm-1 can be assigned to the 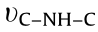 or
or  [17,23]. The broad peaks centered at the range of 3000 to 3500 cm-1 can be assigned to the
[17,23]. The broad peaks centered at the range of 3000 to 3500 cm-1 can be assigned to the 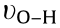 and
and 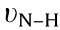 , [24,25]. For UCNS and P-UCNS, the signals at 1463 and 1637 cm-1 (assigned to C=N and C–N, respectively) shift to 1466 and 1639 cm-1 , respectively. In addition, the peak positioned at 1570 cm-1 , ascribed to C=N in the CN heterocycles, becomes inconspicuous, implying that g-C3N4 were protonated successfully [15,26,27]. The new signal observed at 985 cm-1 in the P-UCNS spectrum, corresponds to the phosphoric acid groups [28]. The FT-IR spectrum of PW12 shows characteristic vibration peaks at 1080, 984, 890, and 798 cm-1 , respectively [29]. All of the characteristic peaks for PW12 and P-UCNS mentioned so far can also be observed in the spectrum of PW12 -P-UCNS. Therefore, the above results indicate that ① the g-C3N4 successfully transforms to P-UCNS after exfoliating, protonating, and modifying by phosphoric acid; ② PW12 is successfully incorporated on the P-UCNS surface; ③ the primary Keggin structure of PW12 remains intact after immobilisation.
, [24,25]. For UCNS and P-UCNS, the signals at 1463 and 1637 cm-1 (assigned to C=N and C–N, respectively) shift to 1466 and 1639 cm-1 , respectively. In addition, the peak positioned at 1570 cm-1 , ascribed to C=N in the CN heterocycles, becomes inconspicuous, implying that g-C3N4 were protonated successfully [15,26,27]. The new signal observed at 985 cm-1 in the P-UCNS spectrum, corresponds to the phosphoric acid groups [28]. The FT-IR spectrum of PW12 shows characteristic vibration peaks at 1080, 984, 890, and 798 cm-1 , respectively [29]. All of the characteristic peaks for PW12 and P-UCNS mentioned so far can also be observed in the spectrum of PW12 -P-UCNS. Therefore, the above results indicate that ① the g-C3N4 successfully transforms to P-UCNS after exfoliating, protonating, and modifying by phosphoric acid; ② PW12 is successfully incorporated on the P-UCNS surface; ③ the primary Keggin structure of PW12 remains intact after immobilisation.
《Fig. 2》

Fig. 2. The (a) XRD and (b) FT-IR for different specimens (UCNS, P-UCNS, bulk g-C3N4, PW12, and PW12-P-UCNS). JCPDS: Joint Committee on Powder Diffraction Standards.
The PW12-P-UCNS photocatalyst was also analysed using XPS. Peaks for P, O, N, W, and C elements are clearly detected for PW12-P-UCNS (Fig. 3(a)). The C 1s XPS spectrum of the PW12-PUCNS (Fig. 3(b)), can be divided into four signals centered at 289.2, 288.0, 286.0, and 284.8 eV. The peaks centered at 288 and 284.8 eV are attributed to the N=C(–N)2 unit and reference C species, respectively [16,30,31]. The peaks originated from C species of C–O and C–NH2 groups can be found at 289.2 and 286.0 eV. In the N 1s spectrum (Fig. 3(c)), the four peaks located at 404.0, 400.2, 399.1, and 398.2 eV are attributed to the C–NH+ =C, C–N–H or N–H2 groups, N–(C)3, and sp2 hybridised C–N=C groups, respectively [32–35]. Moreover, it is clear that the N 1s and C 1s spectra of PW12-P-UCNS show an obvious shift, compared with UCNS and P-UCNS due to the protonation and strong electrostatic interaction of the PW12 species [13,17,29]. The W 4f XPS spectrum of the PW12-P-UCNS (Fig. 3(d)) shows that it is divided into two signals centered at 37.4 and 35.3 eV, ascribing to the W 4f5/2 and W 4f7/2 spin–orbit components accordingly, indicating the WVI species of the incorporated PW12 [36]. Moreover, the negative shifts of the binding energy that can be observed compared with the PW12 spectrum (W 4f5/2 of 37.9 eV and W 4f7/2 of 35.8 eV), can be explained through the chemical interaction between P-UCNS and the Keggin units [37].
《Fig. 3》
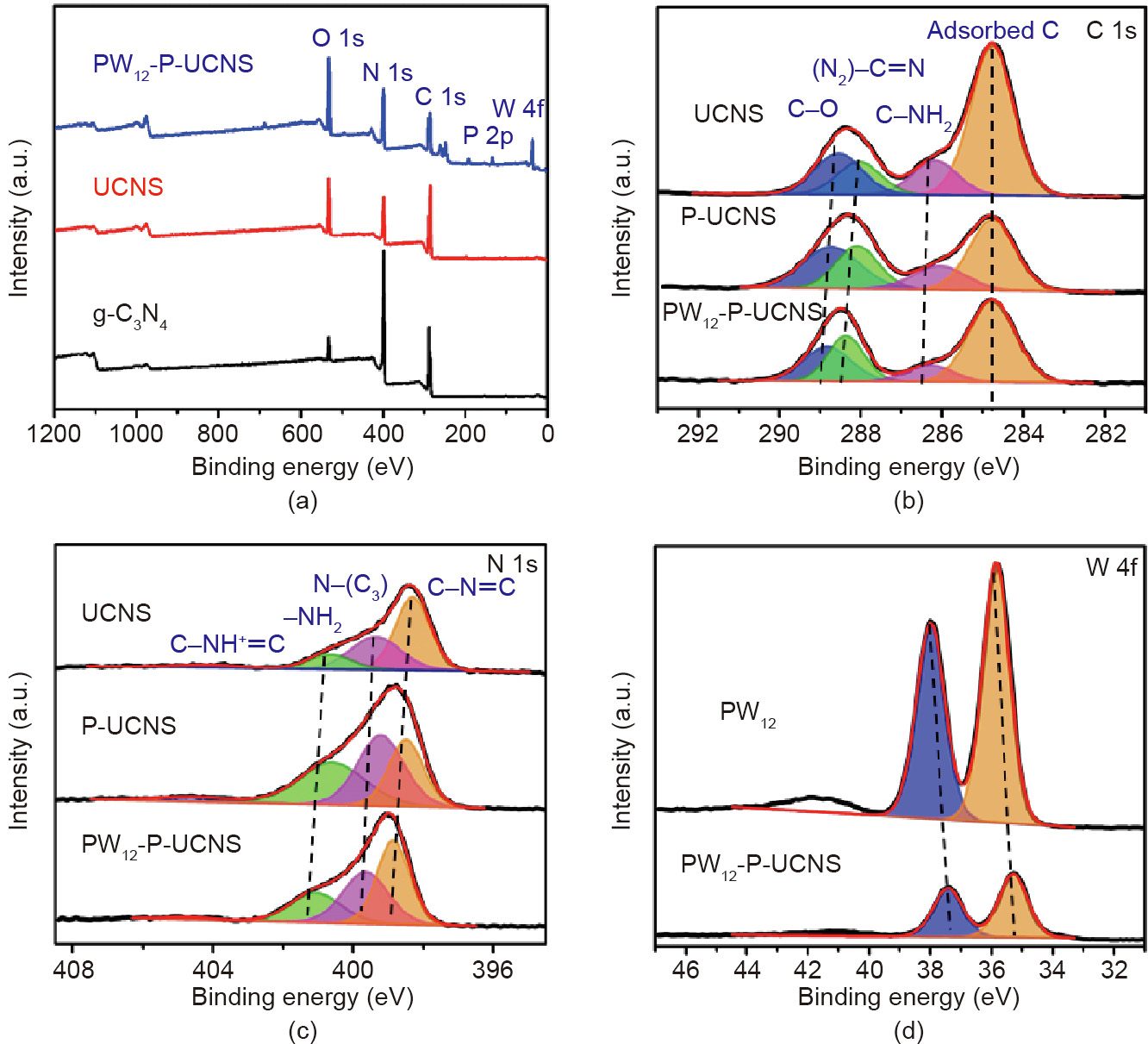
Fig. 3. (a) XPS survey of different samples (PW12-P-UCNS, g-C3N4, and UCNS); XPS spectra of the (b) C 1s, (c) N 1s of UCNS, P-UCNS, and PW12-P-UCNS, and (d) W 4f for PW12 and PW12-P-UCNS.
《3.3. Morphological characteristics and textural properties》
3.3. Morphological characteristics and textural properties
The morphology of PW12-P-UCNS was investigated using TEM and was compared with g-C3N4, UCNS, and P-UCNS. As shown in Fig. 4, all the prepared materials show layered nanostructures. Compared with g-C3N4, which shows thick and blocky nanostructure (~29 nm for thickness, in Appendix A Fig. S1), the as-prepared PW12-P-UCNS exhibits extremely thin nanosheets (~12 nm for thickness, Fig. S1). The open-up surface provides abundant active sites and shorter diffusion distances, is conducive to accelerate charge separation and mass transfer, and thereby leads to enhanced photo-oxidation activity of PW12-P-UCNS [38]. Moreover, it can be seen that the thickness of UCNS rapidly decreases to 10 nm following the hydrothermal treatment of g-C3N4, and it almost remains after the incorporation of phosphoric acid and POMs. This observation is consistent with the result obtained from atomic force microscopy (AFM). Closer inspection of the HRTEM images (Fig. S2 in Appendix A) shows that the prepared PW12-P-UCNS is well-defined with uniformly dispersed black spots. This is in agreement with the dimensions of the PW12 clusters, and suggests that the clusters are uniformly incorporated on P-UCNS. Furthermore, the as-prepared UCNS, P-UCNS, and PW12-P-UCNS exhibit porous nanostructures, as revealed by the measurement of porosity (Fig. S3 in Appendix A).
《Fig. 4》

Fig. 4. TEM of (a) PW12-P-UCNS, (b) P-UCNS, (c) UCNS, and (d) bulk g-C3N4.
《3.4. Optical absorption properties》
3.4. Optical absorption properties
The energy band features and optical absorption properties of UCNS, g-C3N4, P-UCNS, and PW12-P-UCNS are discussed based on the obtained UV–visible diffuse reflectance spectra (UV–Vis/DRS). In Fig. 5, the absorption region of PW12 is within the area of 200 to 422 nm, attributed to the charge transfer from O 2p to W 5d orbit at W=O and W–O–W, respectively [12]. Moreover, it can be observed that the absorption edge of other materials ends up in the scope of 440–480 nm because of the electron transition from the valence band (VB) contributed by N 2p to the conduction band (CB) contributed by C 2p orbitals [39]. Furthermore, the absorption peak associated with PW12 or P-UCNS could not be observed in the spectra of PW12-P-UCNS (Fig. 5), indicating that the incorporated PW12 is uniformly dispersed throughout the whole composite [13]. According to the plots of  vs photon energy (hv) of Fig. S4, the band gap (Eg) of various materials can be estimated from Tauc plots expressed by
vs photon energy (hv) of Fig. S4, the band gap (Eg) of various materials can be estimated from Tauc plots expressed by  , where v, Eg, A, and α are the frequency of light, the energy of band gap, proportionality constant, and absorption modulus, respectively [40,41]. The Eg of PW12, g-C3N4, UCNS, and P-UCNS are 2.80, 2.62, 2.69, and 2.42 eV, respectively. Comparing with g-C3N4 and UCNS, the blue shift of the band gap energy proves that the light absorption capacity of P-UCNS is improved.
, where v, Eg, A, and α are the frequency of light, the energy of band gap, proportionality constant, and absorption modulus, respectively [40,41]. The Eg of PW12, g-C3N4, UCNS, and P-UCNS are 2.80, 2.62, 2.69, and 2.42 eV, respectively. Comparing with g-C3N4 and UCNS, the blue shift of the band gap energy proves that the light absorption capacity of P-UCNS is improved.
《Fig. 5》

Fig. 5. UV–Vis/DRS plots of different samples (g-C3N4, UCNS, P-UCNS, PW12, and PW12-P-UCNS).
《3.5. Performance studies》
3.5. Performance studies
In order to test the performance of PW12-P-UCNS, the oxidation reaction of benzyl alcohol under Xe lamp illumination was chosen. For comparison, PW12, P-UCNS, and PW12-P-UCNS with varying PW12 loading were also tested (the corresponding characterization results can be seen in Figs. S5–S7).
Fig. 6(a) shows the activity of PW12, P-UCNS, and PW12-P-UCNS towards the selective benzyl alcohol oxidation under Xe lamp illumination. A blank experiment without photocatalyst was also performed under the same conditions, and the results showed that the reaction without PW12-P-UCNS, under irradiation, for 2 h, barely proceeds. The adsorption of reactants on as-prepared photocatalysts was also considered, by initially leaving the catalytic system in the dark, under stirring, for 0.5 h. After illumination for 2 h, the order of photo-oxidation activity follows the order of PW12- P-UCNS > P-UCNS > PW12. Obviously, the as-prepared PW12-PUCNS photocatalyst exhibits the highest photocatalytic oxidation activity, reaching benzyl alcohol conversion of 58.3% and benzaldehyde selectivity of 99.5%. The influence of PW12 loading of PW12-PUCNS on photocatalytic oxidation activity was also researched. As shown in Fig. 6(b), increasing PW12 loading leads to an increase of the conversion to benzyl alcohol, while the selectivity to benzaldehyde markedly decreases. In order to obtain high conversion to benzyl alcohol and selectivity to benzaldehyde, PW12-P-UCNS with 34.8 wt% PW12 loading was selected for subsequent photocatalytic tests with comprehensive consideration of conversion-to-benzyl alcohol and selectivity-to-benzaldehyde (Fig. S8). Moreover, the oxidation activity of as-prepared PW12-P-UCNS was compared with the reported value in literature and exhibited excellent catalytic performance (Table S1). The prepared PW12-P-UCNS shows the following competitive advantages: ① The solvent of catalytic system is water, making the process green, eco-friendly, and sustainable; ② high target product selectivity and reactant conversion obtained under mild reaction conditions (short reaction time and low reaction temperature); ③ high stability and inexpensive production compared to noble metals.
《Fig. 6》

Fig. 6. The catalytic performance of (a) various photocatalysts and (b) PW12-P-UCNS with different PW12 loading towards the benzyl alcohol conversion and benzaldehyde selectivity in deionized water. PW12-P-UCNS 1, PW12-P-UCNS 2, PW12-P-UCNS, and PW12-P-UCNS 3 represent PW12 loading of 13.7%, 22.6%, 34.8%, and 46.2%, respectively. Reaction conditions: 20 mg catalysts, 10 mL deionized water, 0.1 mmol benzyl alcohol, and 2 h Xe lamp irradiation.
《3.6. Photocatalyst reusability》
3.6. Photocatalyst reusability
The regeneration and reusability of photocatalysts are considered as two key issues to evaluate catalytic performance. Herein, the reusability of as-prepared PW12-P-UCNS was evaluated by catalysing the oxidation of benzyl alcohol for five consecutive cycles. As shown in Fig. 7(a), after five consecutive cycles, the benzaldehyde selectivity is retained almost as high as in the original performance, whereas the benzyl alcohol conversion shows a slight decrease after three cycles. In order to investigate the cause of activity loss, we initially performed inductively coupled plasma atomic emission spectrometry (ICP-AES) analysis using the reaction liquid after separating the photocatalyst, to identify whether PW12 is leaching in the catalytic system. Based on tungsten content results, it can be confirmed that leaching of PW12 species hardly occurs in the examined reaction system. Furthermore, structural and morphological information of the used PW12-P-UCNS catalyst was obtained using FT-IR, XRD, and TEM. As can be seen in Fig. 7(b), the used PW12-P-UCNS catalyst exhibits thin flaky nanostructure. Moreover, the characteristic signals ascribed to the Keggin units of PW12 can still be found and there is no obvious structural change in Fig. 7(d). These results suggest that the activity loss of PW12-P-UCNS after five consecutive cycles could be attributed to the mass loss of photocatalyst during recycling, although for at least five consecutive cycles, the as-prepared PW12- P-UCNS does not show any morphological or structural change.
《Fig. 7》

Fig. 7. (a) Reusability; (b) the image of TEM; (c) spectra of FT-IR; and (d) spectra of XRD towards fresh and used PW12-P-UCNS photocatalyst. Reaction conditions: 20 mg catalysts, 10 mL deionized water, 0.1 mmol benzyl alcohol, and 2 h Xe lamp irradiation.
《3.7. Photocatalytic mechanism》
3.7. Photocatalytic mechanism
The behaviors of photogenerated electron-hole pairs can be monitored by testing their photoelectrochemical properties [4]. Initially, the separation and transfer efficiency of the photogenerated charge carriers towards the as-prepared P-UCNS and PW12- P-UCNS photocatalysts was evaluated using transient photocurrent measurement. As shown in Fig. 8(a), the photocurrent responses of P-UCNS and PW12-P-UCNS can be reproduced and are stable over a period of five illumination cycles. It was found that PW12-P-UCNS (2.52 μA) showed a 3.8-fold enhancement in the photocurrent intensity compared with P-UCNS (0.66 μA). This indicates the much-improved separation and transfer efficiency for photogenerated electrons and holes, attributed to the incorporation of PW12 species. The incorporated PW12 functions as superior electron acceptors, restricting the recombination of photogenerated electrons and holes. Moreover, the intensity of the photoluminescence (PL) spectra emission spectra indicates the recombination rate of photogenerated electron-hole pairs. As shown in Fig. S9, the asprepared PW12-P-UCNS exhibited the lowest radiation recombination rate of photogenerated carriers compared with g-C3N4, UCNS and P-UCNS, which was consistent with the results obtained from transient photocurrent measurement.
Subsequently, the separation efficiency of the photogenerated electrons and holes was investigated using electrochemical impedance spectroscopy (EIS) measurements [2]. The high-frequency semicircle in the Nyquist plot represents the charge-transfer procedure, whose radius reflects the charge-transfer resistance [42,43]. The smaller radius of semicircle indicates the smaller charge-transfer resistance. The as-prepared PW12-P-UCNS showed smaller radius of curvature compared with P-UCNS, implying higher charge separation efficiency (Fig. 8(b)). The above results show that the charge separation efficiency and resistance of charge transfer of photocatalyst were greatly improved after the incorporation of PW12.
《Fig. 8》

Fig. 8. Profiles of (a) photocurrent-time and (b) electrochemical impedance spectroscopy (EIS) measurement. –Z'': negative imaginary impedance; Z' : real impedance.
In order to investigate the potential mechanism of PW12-PUCNS nanocomposite for selective alcohol oxidation, we examined the main active species in the reaction by the 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) spin-trapping electron spin resonance (ESR) measurements. As shown in Fig. 9(a), there was no obvious signal without light irradiation. Under solar light and the presence of PW12-P-UCNS photocatalyst, there were characteristic signals with 1:1:1:1 of intensity ratio in methanol dispersion, which can be assigned to the DMPO-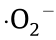 adduct [44]. This indicates that
adduct [44]. This indicates that 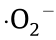 can be formed in the photocatalytic course upon irradiation.
can be formed in the photocatalytic course upon irradiation.
The effect of different active species on the model reaction was carefully investigated by using potassium persulfate (K2S2O8) as electron scavengers, oxalic acid (C2H2O4) as hole scavengers, 1,4- benzoquinone (BQ) as 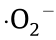 scavengers, and tertbutyl alcohol (TBA) as
scavengers, and tertbutyl alcohol (TBA) as 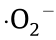 scavengers [1,2,5,45]. As shown in Fig. 9(b), when C2H2O4 or BQ was introduced, the conversion of benzyl alcohol rapidly declined, demonstrating that
scavengers [1,2,5,45]. As shown in Fig. 9(b), when C2H2O4 or BQ was introduced, the conversion of benzyl alcohol rapidly declined, demonstrating that 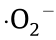 and holes were important active moieties in the current oxidation system. Meanwhile, the decrease of conversion-to-benzyl alcohol in the TBA-added photocatalytic system was negligible, implying that ·OH radicals showed no vital role during the current photocatalytic reaction process. Furthermore, it was found that conversion-to-benzyl alcohol increased slightly after adding K2S2O8. This suggested that the addition of electron scavenger increased greatly the consumption of electrons, thus increasing the amount of active holes on PW12-P-UCNS and improving the indirect hole oxidation of benzyl alcohol on the surface [1].
and holes were important active moieties in the current oxidation system. Meanwhile, the decrease of conversion-to-benzyl alcohol in the TBA-added photocatalytic system was negligible, implying that ·OH radicals showed no vital role during the current photocatalytic reaction process. Furthermore, it was found that conversion-to-benzyl alcohol increased slightly after adding K2S2O8. This suggested that the addition of electron scavenger increased greatly the consumption of electrons, thus increasing the amount of active holes on PW12-P-UCNS and improving the indirect hole oxidation of benzyl alcohol on the surface [1].
《Fig. 9》
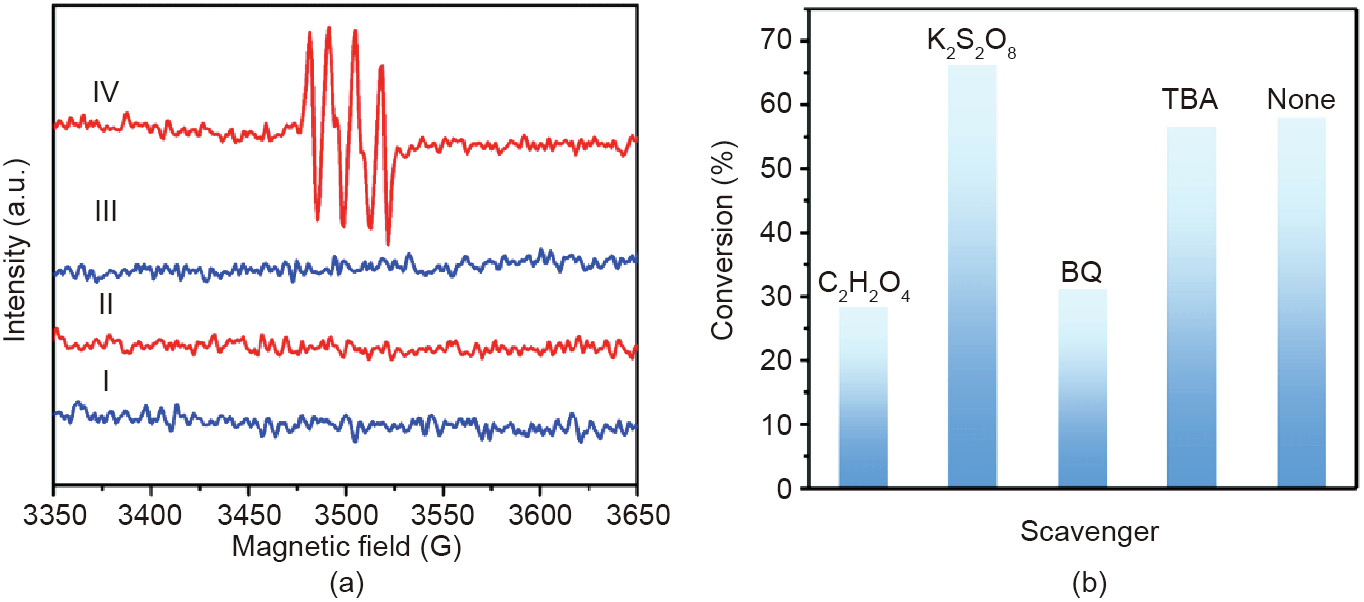
Fig. 9. (a) The DMPO spin-trapping ESR spectra of PW12-P-UCNS. I: DMPO in dark; II: PW12-P-UCNS in dark; III: DMPO-Xe lamp; IV: PW12-P-UCNS-Xe lamp. (b) The results of different scavengers in the PW12-P-UCNS-photocatalytic benzyl alcohol oxidation. 1G = 1 × 104 T.
Therefore, it was proven that  and
and 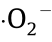 were the main active moieties for the examined oxidation system under solar light illumination.
were the main active moieties for the examined oxidation system under solar light illumination.
In order to estimate the relative positions of CB and VB of PW12 and P-UCNS, we calculated the optical band gap, CV, and measured the flat band potential (Fig. S10). Based on the above results, the conventional type II-heterojunction and direct Z-scheme mechanism can be used to describe the current catalytic system.
If the as-prepared PW12-P-UCNS followed the type IIheterojunction mechanism, the electrons in the CB of P-UCNS could be accumulated in the CB of PW12, while the holes in the VB of PW12 could transfer to the VB of P-UCNS. As shown in Fig. S11, the CB level of PW12 (+0.30 V vs NHE, determined by CV, NHE is the normal hydrogen electrode, Fig. S10) was more positive than the redox potential of O2/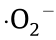 (0.33 V vs NHE) [28,46], which was hardly to reduce O2 to
(0.33 V vs NHE) [28,46], which was hardly to reduce O2 to 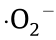 radicals directly. In addition, the holes on the VB of P-UCNS were also unable to generate
radicals directly. In addition, the holes on the VB of P-UCNS were also unable to generate 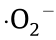 radicals. The above type II-heterojunction mechanism conflicted with the results of ESR. Therefore, a light-driven Z-scheme was proposed instead, to describe the system.
radicals. The above type II-heterojunction mechanism conflicted with the results of ESR. Therefore, a light-driven Z-scheme was proposed instead, to describe the system.
As illustrated in Fig. 10, a reasonable mechanism of PW12-P-UCNS selectively photocatalyzed benzyl alcohol oxidation to produce benzaldehyde was revealed. The electron-hole pairs are generated after irradiation of the PW12-P-UCNS (Eq. (1)). Because of the close integration between PW12 and P-UCNS as well as well-matched energy band structure, the initial transfer of electrons from the CB of the PW12 species to the VB of P-UCNS was facilitated, and thus they could further realize the recombination with the holes on the VB of P-UCNS. During the Z-scheme-driven photo-oxidation process of benzyl alcohol, the benzyl alcohol cation radicals (C6H5CH2OH*+ ) were initially produced by the holes on the VB of PW12 (Eq. (2)). Meanwhile, the electrons of P-UCNS are more likely to be scavenged by molecular oxygen to produce more abundant 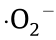 radicals (0.33 V vs NHE) (Eq. (3)), which benefits the modification of phosphoric acid and thereby improves the adsorption of O2 on the surface of P-UCNS [13,28,46]. Lastly, the activated intermediates further react with produced
radicals (0.33 V vs NHE) (Eq. (3)), which benefits the modification of phosphoric acid and thereby improves the adsorption of O2 on the surface of P-UCNS [13,28,46]. Lastly, the activated intermediates further react with produced 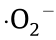 to generate the targeted benzaldehyde (Eq. (4)).
to generate the targeted benzaldehyde (Eq. (4)).
On the other hand, as-prepared PW12-P-UCNS photocatalyst can act as the H-bonding acceptor because of the C–N–H, C–OH, and POMs species present on the surface. Accordingly, the surface of PW12-P-UCNS photocatalysts prefers to adsorb the reactants via H-bonding or electrostatic interaction, leading to the high benzyl alcohol conversion. Meanwhile, the weak interaction between benzaldehyde and PW12-P-UCNS makes the former desorb quickly from the surface, avoiding the over-oxidation of the target product and thereby obtaining high selectivity of benzaldehyde. Such a Z-scheme-driven photocatalysedoxidation reaction shows excellent benzyl alcohol conversion and benzaldehyde selectivity attributed to the sharp separation of photogenerated electron-hole pairs and abundance of active species of 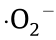 radicals. Based on the aforementioned results, as-prepared PW12-P-UCNS can be used as an effective and environmentally benign photocatalyst for the chosen oxidation reaction.
radicals. Based on the aforementioned results, as-prepared PW12-P-UCNS can be used as an effective and environmentally benign photocatalyst for the chosen oxidation reaction.
《Fig. 10》

Fig. 10. The proposed mechanism for the PW12-P-UCNS-catalysed benzyl alcohol photo-oxidation under the current catalytic system.
《4. Conclusion》
4. Conclusion
To summarise, an efficient and environmentally friendly photocatalyst (PW12-P-UCNS) was successfully constructed by immobilising PW12 on g-C3N4 nanosheets modified using phosphoric acid. The as-prepared PW12-P-UCNS photocatalyst showed excellent photocatalytic performance including its activity and stability in selective benzyl alcohol oxidation to produce benzaldehyde, that is, 58.3% conversion of benzyl alcohol and 99.5% selectivity to benzaldehyde within 2 h. Careful investigation of the photoelectrochemical properties, including photocurrent, scavenger experiments, and ESR measurement, such a Z-schemedriven catalytic performance can be assigned to the following facts. Firstly, the incorporated Keggin units facilitate the spontaneous migration of electrons, and thereby accelerate interfacial charge carrier separation; secondly, more abundant 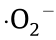 radicals (the detected main active species during the current selective photooxidation reaction process) are generated, which is beneficial from the improvement of O2 adsorption on the surface of P-UCNS. Based on the aforementioned results and observations, the PW12-P-UCNS photocatalysts show great potential for driving chemical oxidation reactions using sunlight.
radicals (the detected main active species during the current selective photooxidation reaction process) are generated, which is beneficial from the improvement of O2 adsorption on the surface of P-UCNS. Based on the aforementioned results and observations, the PW12-P-UCNS photocatalysts show great potential for driving chemical oxidation reactions using sunlight.
《Acknowledgements》
Acknowledgements
This research was supported by the National Nature Science Foundation of China (21625101, 21521005, and 21808011), the National Key Research and Development Program of China (2017YFB0307303), Beijing Natural Science Foundation (2202039) and the Fundamental Research Funds for the Central Universities (XK1802-6, XK1902, and 12060093063).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Lifu Wu, Sai An, and Yu-Fei Song declare that they have no conflict of interest or financial conflicts to disclose.
《Appendix A. Supplementary data》
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eng.2020.07.025.













 京公网安备 11010502051620号
京公网安备 11010502051620号




