《1. Introduction》
1. Introduction
During the period of time in which 5-fluorouracil with leucovorin was the mainstay therapeutic regimen for patients with metastatic colorectal cancer (mCRC), the median overall survival (OS) was stagnant at approximately 8–12 months [1]. With the introduction of oxaliplatin and irinotecan in 2000, the combination regimens 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX) and 5-fluorouracil/leucovorin plus irinotecan (FOLFIRI) became standard therapies in first-line treatment [2]. Moreover, targeted biological therapies that selectively acted on crucial pathways in cancer progression, such as anti-vascular endothelial growth factor (VEGF) or anti-epidermal growth factor receptor (EGFR) agents, further improved life expectancy [3–5].
Another advancement in the treatment of mCRC was the increased recognition that surgically resecting liver-limited metastases to achieve no evidence of disease (NED) may possibly achieve long-term survival outcomes [6,7]. Approximately 30%–50% of mCRC patients present with liver metastasis at the time of diagnosis or disease relapse, and 15%–25% of these patients are potential surgical candidates [7–9]. Retrospective studies in patients who underwent R0 hepatectomy suggest five-year OS rates exceeding 50% [10,11]. In addition, there is increased evidence on the use of radiofrequency ablation (RFA) and stereotactic body radiation therapy (SBRT) as reasonable, alternative options for non-surgical candidates and for those with disease recurrence after hepatectomy [12,13]. Moreover, local treatment strategies for mCRC liver metastases have been commonly applied to manage lung metastases [14–16]. Still, there is a lack of causal evidence from randomized studies demonstrating the efficacy of local therapy on colorectal liver or lung metastases, especially in the era of targeted therapy, as randomized trials addressing this issue are neither ethical nor feasible at present.
With the aforementioned improvements in managing mCRC, changing treatment patterns have been observed among patients in North America and some European countries [17,18]. In China, there is a substantial disparity between real-world clinical practice and standard-of-care guidelines for managing patients with mCRC [19]; meanwhile, survival outcomes of mCRC patients are poorer in China than in developed countries [20,21]. However, owing to the lack of high-quality, large-scale, real-world evidence from mCRC patients in China, it remains unclear whether efforts to improve the quality of care for mCRC would improve patient survival and narrow this gap. In this context, we conducted a large-scale retrospective cohort study to investigate temporal changes in the systemic and local treatment patterns for mCRC at Sun Yat-sen University Cancer Center, and to determine whether these changes were associated with improved patient survival outcomes over time.
《2. Methods》
2. Methods
《2.1. Study population and data extraction》
2.1. Study population and data extraction
This research was a single-institution, retrospective cohort study. Eligible patients were aged 18 or older with histologically confirmed mCRC at Sun Yat-sen University Cancer Center. The detailed patient inclusion and exclusion process is shown in Fig. S1 in Appendix A.
The examined covariates included age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, year of diagnosis, primary tumor location, metastatic site, mutation status of Kirsten rat sarcoma viral oncogene homolog (Kras) or neuroblastoma rat sarcoma viral oncogene homolog (Nras) exon 2–4 and V600E mutation of the B-type Raf kinase gene (BrafV600E), and treatment information on surgery, RFA, SBRT, and systemic therapy. All this information was extracted intelligently from the Big-Data Alliance for Colorectal Cancer (BACC) platform (YiduCloud Technology Ltd., China). The BACC platform is an intelligent big-data platform that integrates and converges large-scale multi-source heterogeneous electronic health-record data from multiple medical centers and hospitals from all over China. On the basis of machine learning and artificial intelligence, the platform structures, normalizes, and integrates information from hospital systems (e.g., admission, discharge, transfer, and surveillance), electronic medical records, and resource information systems including the results of radiological and nuclear imaging, endoscopy, ultrasound, electrocardiography, and pathology and laboratory tests; reorganizes essential data into a table format; and helps in visualizing patients’ diagnosis and treatment data along the time axis, thus facilitating routine clinical practice and scientific research.
All patients provided written informed consent for the storage and use of their information in the hospital database. Study approval was obtained from the independent ethics committees at Sun Yat-sen University Cancer Center. The study was undertaken in accordance with the ethical standards of the World Medical Association Declaration of Helsinki.
《2.2. Outcome measurement》
2.2. Outcome measurement
The outcomes of interest included OS and temporal changes in the utility rate of the following systemic and local therapies: ① cytotoxic agents (i.e., fluoropyrimidines, oxaliplatin, and irinotecan); ② targeted therapies (i.e., cetuximab, bevacizumab, and/or regorafenib); ③ surgical resection, RFA, and/or SBRT to liver, lung, or extrahepatic and/or extrapulmonary metastases; ④ local therapy as the initial course of treatment for mCRC; and ⑤ systemic therapy followed by local therapy. We also assessed the temporal changes in the percentage of patients in whom NED (i.e., elimination or remission of all gross lesions) was achieved after systemic and/or local therapies. Treatment information was collected from the date of initial diagnosis with mCRC to the date of the last visit to the inpatient or outpatient departments at our center. OS was measured from the date of initial diagnosis with mCRC to the date of death from any cause or last follow-up.
《2.3. Statistical analysis》
2.3. Statistical analysis
The 95% confidence interval (CI) for the utility rate of given therapy was estimated using the Clopper–Pearson method. Survival curves were generated using the Kaplan–Meier method, with comparisons between groups performed using the log-rank test. The association between the variable of interest and OS outcomes was indicated by the hazard ratio (HR) and corresponding 95% CI obtained from the multivariable Cox proportional hazards model adjusted for baseline covariates, including age, sex, performance status, primary tumor location, number of metastatic sites, and molecular subtype.
Statistical significance was set as P < 0.05 in a two-tailed test. All statistical analyses were performed using R software version 3.6.1↑ . (↑ http://www.r-project.org)
Descriptive metrics of OS include median OS duration (95% CI) and five-year OS probability (95% CI).
《3. Results》
3. Results
《3.1. Patient characteristics and treatment exposure》
3.1. Patient characteristics and treatment exposure
Between July 2012 and December 2018, a total of 3403 eligible mCRC patients were included in the study (Fig. S1). The baseline characteristics of the patients are summarized in Table S1 in Appendix A. The majority of the patients were male, were younger than 65 years old, had moderately impaired performance status, had left-sided tumors, or had liver metastases. Rat sarcoma viral oncogene homolog (Ras)/Braf mutation status was evident in 34.3% of the patients, the majority of whom had Ras-mutated disease.
The median interval between the date of initial diagnosis with mCRC and the date of initial local or systemic treatment for mCRC at our center was 1.0 month (interquartile range, 0.5–3.4). During the entire treatment course for mCRC, 43.4% of the patients received local therapy (i.e., surgical resection, RFA, and/or SBRT) to the liver (32.0%), lung (12.1%), or extrahepatic and/or extrapulmonary metastases (7.1%). NED was achieved in 948 patients (27.9% of the entire cohort and 64.2% of those receiving local therapy). For patients who received systemic treatment (n = 2978), the therapeutic agents (utility rates) included fluoropyrimidines (99.2%), oxaliplatin (94.1%), irinotecan (56.7%), bevacizumab (38.5%), cetuximab (17.5%), regorafenib (4.8%), and others (9.3%).
《3.2. Survival outcomes with or without local therapy》
3.2. Survival outcomes with or without local therapy
With a median follow-up of 32.8 months (95% CI, 31.5–33.9), the median OS for the entire cohort was 42.8 months (95% CI, 40.7–46.6; Fig. 1(a)). For patients treated with systemic therapy only, the median OS was 25.6 months (95% CI, 24.7–26.9); for those receiving local therapy, the median OS was not reached (NR) (95% CI, 78.6 months–NR) and the five-year OS probability was 59.8% (95% CI, 56.0%–63.9%). The five-year OS probability was 61.0% (95% CI, 56.9%–65.3%) among patients receiving local therapy limited to liver and/or lung lesions and 53.5% (95% CI, 43.9%–65.3%) among those receiving local therapy to extrahepatic and/or extrapulmonary metastases (Fig. S2 in Appendix A). Moreover, for patients in whom NED was achieved, the five-year OS probability reached 72.8% (95% CI, 68.7%–77.2%; Fig. 1(b)).
《Fig. 1》
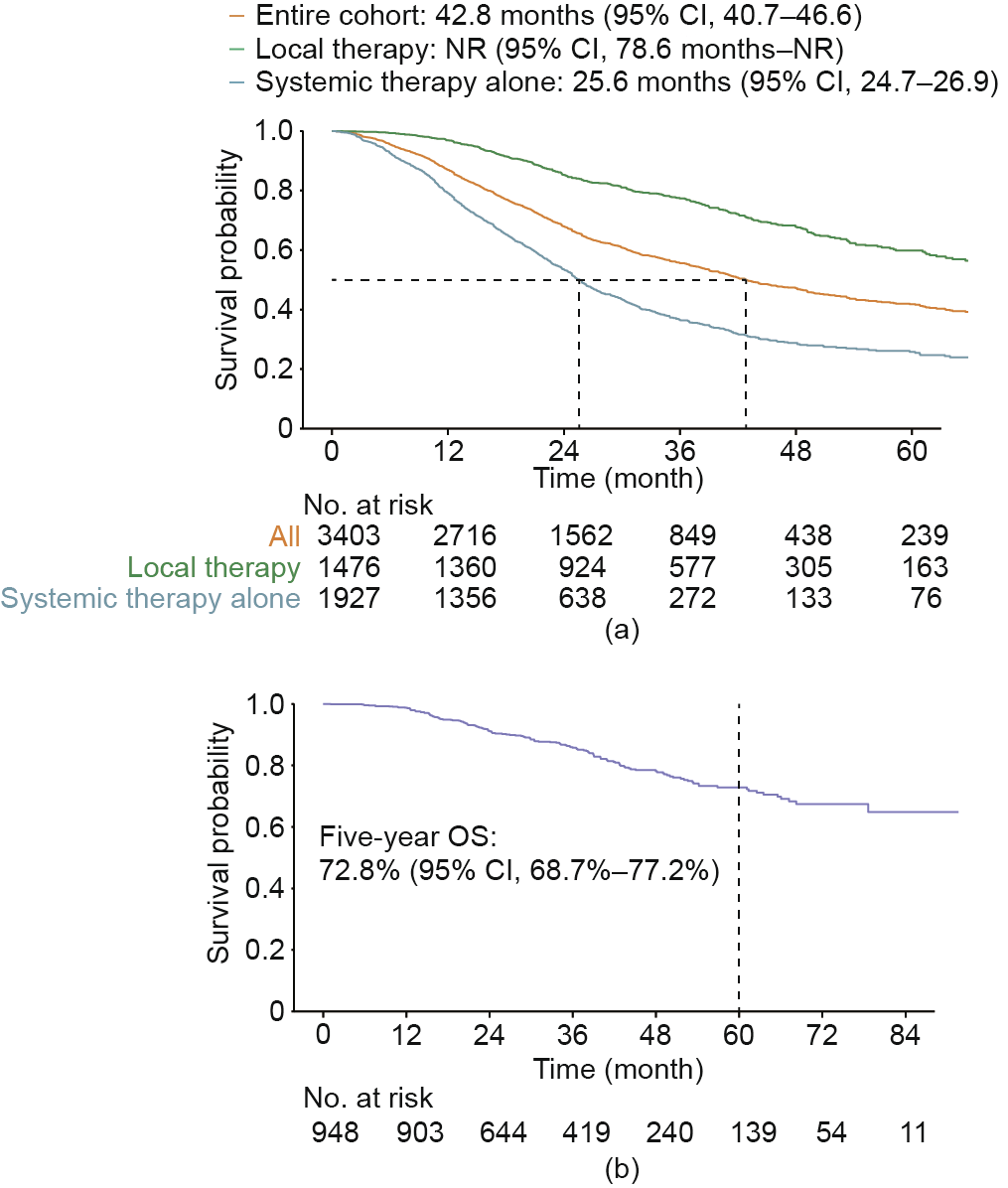
Fig. 1. OS of (a) the entire cohort, patients receiving local therapy, and those receiving systemic therapy only; and (b) patients in whom no evidence of disease was achieved. NR: not reached.
《3.3. Temporal changes in patterns of systemic and local therapy》
3.3. Temporal changes in patterns of systemic and local therapy
To guarantee comparable sample sizes for each group, patients were classified into the following temporal groups according to the year of diagnosis: 2012–2014 (n = 1044), 2015–2016 (n = 1135), and 2017–2018 (n = 1224). Because of the large sample size, statistically significant imbalances between different temporal groups were detected in performance status and molecular subtype; however, the absolute differences were numerically small (Table S2 in Appendix A).
As shown in Fig. 2(a), the percentage of those receiving all three cytotoxic agents (i.e., fluoropyrimidines, oxaliplatin, and irinotecan) during the treatment course was above 50% among all three temporal groups. Notably, the utility rate of either cetuximab or bevacizumab increased modestly from 39.9% (95% CI, 36.7%–43.2%) in 2012–2014 to 43.2% (95% CI, 40.0%–46.4%) in 2015–2016, but then increased dramatically to 60.3% (95% CI, 57.3%–63.2%) in 2017–2018. Similar trends were observed for both cetuximab and bevacizumab. The utility rate of regorafenib also increased continuously over time.
As shown in Fig. 2(b), the utility rate of local therapy increased continuously over time, from 37.9% (95% CI, 35.0%–41.0%) in 2012– 2014 to 44.6% (95% CI, 41.7%–47.5%) in 2015–2016 and 46.9% (95% CI, 44.1%–49.7%) in 2017–2018. The utility rate of local therapy to liver metastases showed a consistent pattern of increase over time, whereas the utility rates of local therapy to lung metastases or extrahepatic and/or extrapulmonary metastases were comparable between the time periods. It is notable that the percentage of patients who received local therapy as the initial course of treatment was comparable among the temporal groups; in contrast, the percentage of patients who received systemic therapy followed by local therapy increased continuously from 17.5% (95% CI, 15.3%– 20.0%) in 2012–2014 to 21.7% (95% CI, 19.3%–24.2%) in 2015–2016, and further reached 28.2% (95% CI, 25.7%–30.8%) in 2017–2018. More importantly, the percentage of patients in whom NED was achieved also increased continuously over time, from 23.4% (95% CI, 20.8%–26.1%) in 2012–2014 to 26.7% (95% CI, 24.1%–29.4%) in 2015–2016 and 32.8% (95% CI, 30.1%–35.5%) in 2017–2018 (Fig. 2(b)).
《Fig. 2》
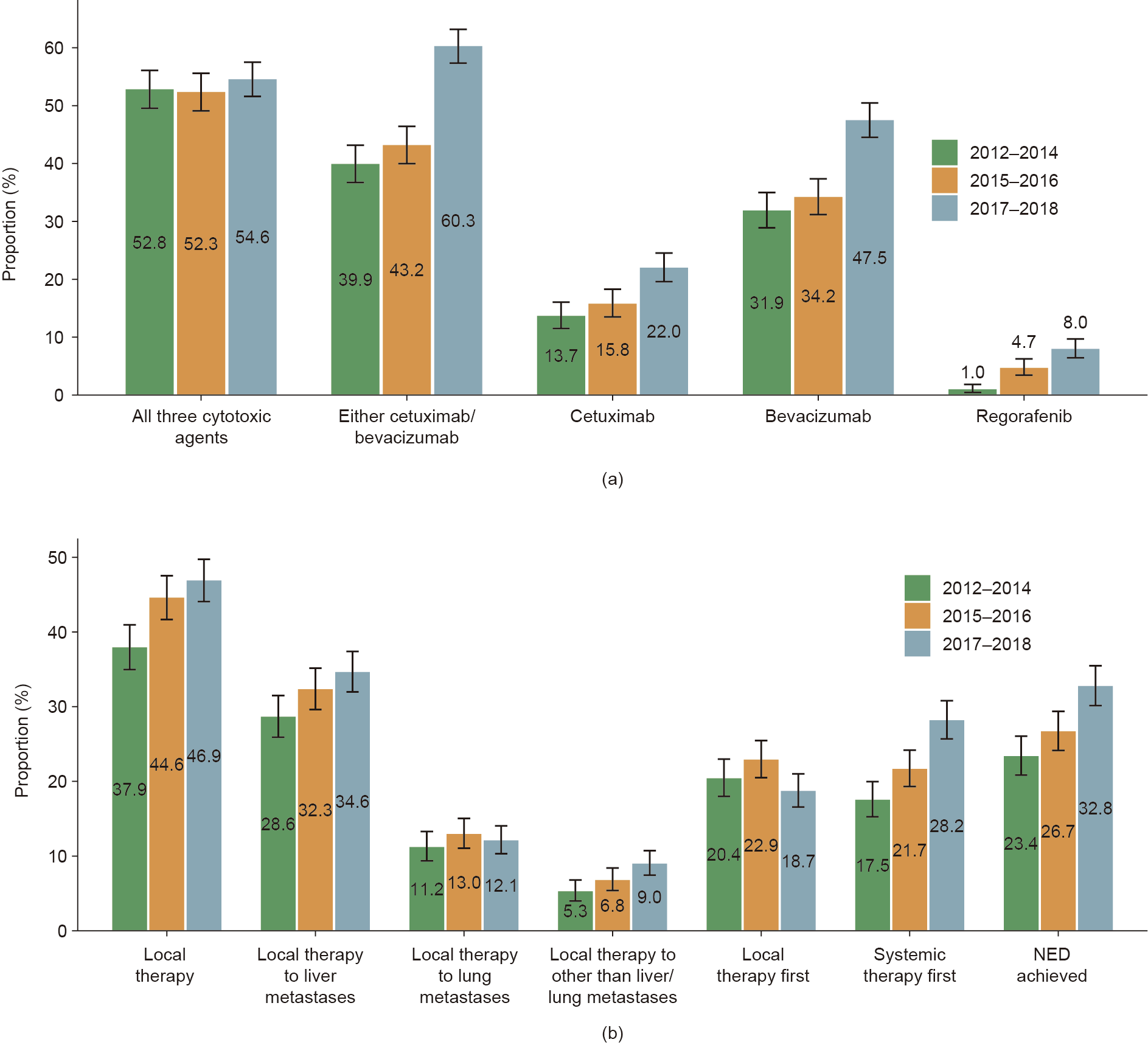
Fig. 2. Temporal changes in (a) systemic treatment and (b) local treatment patterns. To guarantee a comparable sample size for each group, patients were classified into the following temporal groups according to year of diagnosis: 2012–2014 (n = 1044), 2015–2016 (n = 1135), 2017–2018 (n = 1224). Only patients that received systemic therapy (n = 2978) were included in the analysis of temporal changes in systemic treatment patterns.
《3.4. Association between temporal changes in treatment patterns and survival outcomes》
3.4. Association between temporal changes in treatment patterns and survival outcomes
In view of the temporal changes in the treatment patterns and median follow-up durations, we further defined the following temporal groups for subsequent survival analyses: ① 2012–2014 (reference group; 65.3 months (95% CI, 63.3–66.7)); ② 2015–2016 (increased utility of local therapy but not targeted therapy; 42.2 months (95% CI, 40.9–42.8)); and ③ 2015–2018 (increased utility of both local therapy and targeted therapy; 27.7 months (95% CI, 27.0–28.7)).
Compared with 2012–2014, the OS was significantly improved in 2015–2016 (42.1 months (95% CI, 37.9–50.0) vs 33.9 months (95% CI, 31.0–38.0); HR = 0.87 (95% CI, 0.78–0.99); P = 0.034; Fig. 3(a)). However, for patients receiving systemic therapy only, the OS did not significantly differ between the two periods (22.9 months (95% CI, 21.1–25.1) vs 24.4 months (95% CI, 22.6– 26.1); HR = 0.99 (95% CI, 0.86–1.14); P = 0.889; Fig. 3(b)).
《Fig. 3》

Fig. 3. OS of patients in 2015–2016 versus that of patients in 2012–2014. (a) Entire population; (b) patients that received systemic therapy only.
Compared with 2012–2014, the median OS was significantly improved and, strikingly, reached 51.6 months (95% CI, 49.1–NR) in 2015–2018 (HR = 0.75 (95% CI, 0.70–0.81); P < 0.001) as shown in Fig. 4(a). Moreover, for patients treated with systemic therapy only, the OS was still significantly improved in 2015–2018 (26.5 months (95% CI, 25.0–29.3)) compared with 2012–2014 (HR = 0.83 (95% CI, 0.77–0.91); P < 0.001) as shown in Fig. 4(b).
《Fig. 4》

Fig. 4. OS of patients in 2015–2018 versus that of patients in 2012–2014. (a) Entire population; (b) patients that received systemic therapy only.
《3.5. Subgroup analysis by primary tumor location》
3.5. Subgroup analysis by primary tumor location
As shown in Fig. S3(a) in Appendix A, the temporal increase in the use of cetuximab was largely limited to left-sided tumors, whereas the increase in the use of bevacizumab over time was more prominent in right-sided tumors. Moreover, the increase in the use of local therapy over time was observed in both the left- and right-sided subgroups (Fig. S3(b)). In 2012–2014 and 2015–2016, the utility rate of local therapy was higher in patients with left-sided tumors than in those with right-sided tumors; this gap diminished over time, and the utility rate of local therapy was comparable between the left- and right-sided subgroups (approximately 45% for both) in 2017–2018 (Fig. S3(b)).
Overall, patients with right-sided tumors exhibited significantly worse median OS than those with left-sided tumors (37.1 months (95% CI, 31.8–42.1) vs 43.4 months (95% CI, 40.7–48.5); HR = 1.21 (95% CI, 1.06–1.37); P = 0.005) as shown in Fig. 5(a). Primary tumor location remained a significant prognostic factor among patients treated with systemic therapy only (21.7 months (95% CI, 19.8– 24.2) vs 26.7 months (95% CI, 25.2–29.9); HR = 1.24 (95% CI, 1.07–1.44); P = 0.005) as shown in Fig. 5(b), whereas OS was comparable in patients with left-sided tumors and those with rightsided tumors who received local therapy (HR = 0.92 (95% CI, 0.70–1.21); P = 0.571) as shown in Fig. 5(c).
《Fig. 5》
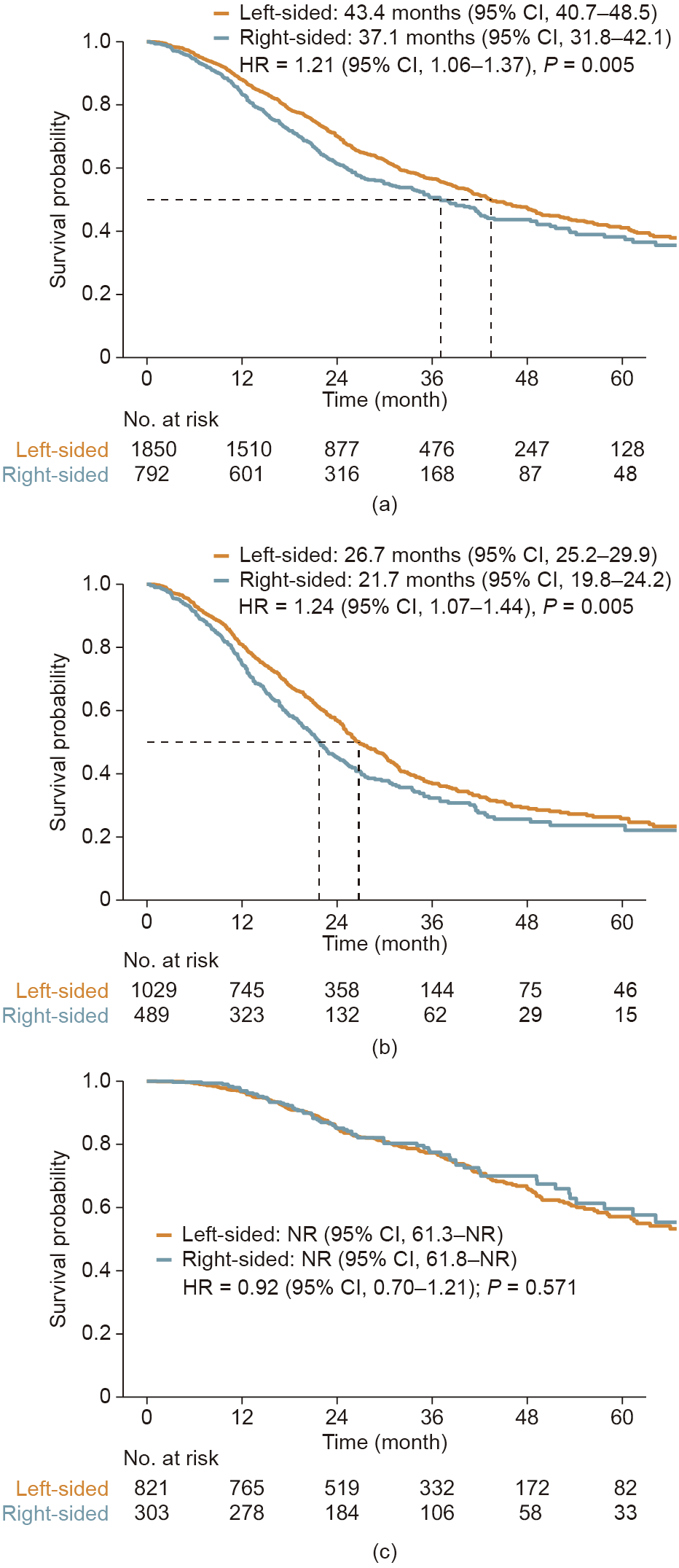
Fig. 5. OS in patients with right-sided tumors versus those with left-sided tumors. (a) Entire population; (b) patients that received systemic therapy only; (c) patients that received local therapy.
《4. Discussion》
4. Discussion
In this study of an mCRC cohort at Sun Yat-sen University Cancer Center, the largest Chinese mCRC cohort thus far with elaborate treatment and outcome information, we demonstrate that the advancements in the treatment of mCRC mainly occurred in two stages, which were coupled with improved survival outcomes. The first stage of improvement was in 2015–2016, with an increased utility rate of local therapy but not targeted therapy. Correspondingly, improved OS was observed for the entire population but not for those receiving systemic therapy only in this period. The second stage of improvement started in 2017, when the utility rate of targeted therapy and that of systemic therapy followed by local therapy substantially increased. Improved OS was also observed for the entire population as well as for those receiving systemic therapy only in 2015–2018. In addition to the observed increase in the utility of local and targeted therapy and multimodality treatment strategies, the improved patient survival may reflect our continuous efforts over the past decade in investigating novel anti-cancer agents and therapeutic strategies and developing clinical practice guidelines and consensus for colorectal cancer (CRC) [22–29].
It is well recognized that only 15%–25% of patients with colorectal liver metastases are eligible for upfront resection [7–9]. Conversion chemotherapy, with or without anti-EGFR or antiVEGF therapies, has been shown to be capable of converting selected patients to resectability, with the conversion rate varying greatly from 13% to above 40% [30–33]. Moreover, local and conversion treatment strategies for colorectal liver metastases have also been commonly applied to managing lung metastases, albeit with scarce relevant evidence. In this present cohort, the utility rate of local therapy (i.e., surgical resection, RFA, and/or SBRT to the liver, lung, or extrahepatic and/or extrapulmonary metastases) as the initial course of treatment was stagnant at around 20% over time, whereas the utility rate of systemic therapy followed by local therapy continued to increase after 2017, with a 47% utility rate of local therapy in 2017–2018. More importantly, with the advancements in systemic and/or local therapies, the percentage of patients in whom NED was achieved increased continuously over time, exceeding 30% in 2017–2018; the five-year OS probability for such patients reached 73% in our cohort, which was comparable to that of patients with liver-limited metastases receiving R0 hepatectomy [10,11]. Altogether, these findings provide compelling evidence of the value of appropriate local therapy to achieve long-term survival outcomes in selected patients; and highlight the importance of multidisciplinary conferencing and cooperation to determine the optimal multimodality treatment strategies, such as the timing of systemic and local therapy and the choice of systemic therapeutic regimens and local treatment approaches, for mCRC patients.
Over the past decade, there has been mounting evidence of the attenuated efficacy of anti-EGFR therapy in right-sided mCRC compared with left-sided tumors [34–37]. Such data have led the North American National Comprehensive Cancer Network (NCCN) guidelines for CRC to recommend against the use of anti-EGFR agents in the first-line treatment of ras wild-type right-sided mCRC since 2017 [38]. Notably, even before this update in the NCCN guidelines, the temporal increase in the use of cetuximab in our cohort was largely limited to those with left-sided tumors, which is reflective of the leading-edge management of mCRC at our institution [39]. Moreover, prior studies have demonstrated that primary tumor location is predictive of survival outcomes for mCRC patients, irrespective of systemic treatment regimens [34,40]. Consistent with these findings, patients with right-sided tumors exhibited significantly worse OS than those with left-sided tumors when treated with systemic therapy only in the current study. Nonetheless, for the patients that received local therapy, OS was comparable between right- and left-sided mCRC patients, which is consistent with previous observations of favorable survival outcomes after curative-intent hepatectomy in patients with right-sided tumors [41,42]. More importantly, although the rate of receiving local therapy was lower in the right-sided subgroup than in the leftsided subgroup before 2017, a similar utility rate of local therapy was achieved in the right- and left-sided subgroups in 2017– 2018 (approximately 45% for both), which could be attributed to the improvement in systemic therapeutic regimens and multidisciplinary management.
Although it has been reported that the Asian CRC population may have longer OS than the Caucasian population [43], prior evidence has shown that the survival outcomes of mCRC were much poorer in China compared with developed countries [17,18]. A possible explanation might be the difference in economic status, as the utility rate of cetuximab and bevacizumab in China—even after being included in the national insurance reimbursement list in 2017—was still lower than that in developed countries [24]. Moreover, a recent multi-institutional study suggested a substantial discrepancy between clinical practice and guidelines for managing mCRC patients in China [19]. It is notable that the present study provides encouraging data showing that the median OS for patients with mCRC has reached 43 months at our institution, which is one of the top two cancer centers in China. When examined separately, the survival outcomes for patients receiving local therapy and for those receiving systemic therapy only both compared favorably with those of other international high-volume centers [10,11,44–46]. The percentage of patients receiving all three cytotoxic agents—an indicator of the ‘‘continuum of care” that has been associated with prolonged OS [47]— has been greater than 50% at our center since 2012, which is comparable to the percentage in the trial setting [48,49]. Taken together, these findings suggest that in-depth education programs and training courses for oncologists are paramount in improving the nationwide quality of care for mCRC patients, as well as in narrowing the gap between China and developed countries.
Our study has several limitations. First, although imaging information, pathology reports, laboratory tests, and pharmaceutical and surgical records from clinical practice at our center are almost entirely captured by the BACC platform, information on the management of mCRC before patients visited our center can only be extracted from the History of Present Illness records, which can result in substantial amounts of missing data for certain variables (e.g., Ras/Braf mutation status). Future advancement in machine learning and artificial intelligence techniques for natural language processing and improvement in front-end electronic medical record systems would hopefully mitigate this challenge. Second, although the BACC platform now covers more than 25 hospitals in China, only the processed data from our center are mature enough for elaborate analyses at present. Future multi-center cohort studies based on this platform are warranted to further assess the generalizability of our findings in other institutions in China. Third, although our time-trend analysis avoids selection bias from retrospectively comparing survival outcomes in patients with or without targeted or local therapy, there might exist some unobserved covariates that were imbalanced between the temporal groups, which may have introduced potential bias to our findings.
《5. Conclusions》
5. Conclusions
This study demonstrates that the clinical management of mCRC patients, as indicated by the utility rate of targeted and local therapies, has been continuously evolving at Sun Yat-sen University Cancer Center, and is associated with continuously improving patient survival outcomes. Our findings document the rationale for promoting multidisciplinary conferencing and cooperation and refining multimodality treatment approaches, as well as providing in-depth education and training for oncologists, in order to improve the nationwide quality of care for managing mCRC patients in China.
《Acknowledgements》
Acknowledgements
This study was supported by the grants from the National Natural Science Foundation of China (81930065), the Natural Science Foundation of Guangdong Province (2014A030312015), the Science and Technology Program of Guangdong (2019B020227002), and the Science and Technology Program of Guangzhou (201904020046, 201803040019, and 201704020228). We appreciate YiduCloud Technology, Ltd. (Beijing, China) for supporting part of the data extraction and processing. We appreciate our native language editor (Mr. Christopher Lavender of Sun Yat-sen University Cancer Center, Guangzhou, China) for his assistance in editing this manuscript.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Zi-Xian Wang, Yi-Chen Yao, Zong-Jiong Mai, Wu-Hao Lin, You-Sheng Huang, Ying Jin, Hui-Yan Luo, Dong-Sheng Zhang, Feng-Hua Wang, Feng Wang, Gong Chen, Pei-Rong Ding, Yun-Fei Yuan, Yu-Hong Li, Jin-Hua Huang, Zhi-Zhong Pan, and Rui-Hua Xu declare that they have no conflict of interest or financial conflicts to disclose.
《Appendix A. Supplementary data》
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eng.2020.10.017.













 京公网安备 11010502051620号
京公网安备 11010502051620号




