《1. Introduction》
1. Introduction
Protein fibers inspired by spider and silkworm silks have been widely explored due to their extraordinary mechanical performance and functionalities [1–7]. With their outstanding intrinsic features, these fibers have promising applications in biomedicines, wound healing, sensors, drug delivery, and tissue engineering [8–12]. Engineered spinning technology has been extensively employed for the generation of protein fibers, including electrospinning [13–15], draw spinning [16], wet spinning (WS) [17], solution blow spinning [18], and dry spinning [19,20]. However, these artificial methods are not very similar to the natural spinning process, and exhibit less control over the length, diameter, and mechanical properties of the as-spun fibers [21,22]. As a highly concentrated protein solution usually serves as a spinning dope during the spinning process, a subsequent physical solidification process for the construction of protein fibers cannot be avoided [23,24]. In addition, the mass production of continuous functional protein fibers is still limited. It is worth noting that the micro-sized spinning ducts of spiders or silkworms can be considered as typical microfluidic devices [25]. Therefore, microfluidics has been explored for engineered spinning and offers great potential for fabricating protein fibers in a simple and controllable way [26,27].
《Fig. 1》

Fig. 1. A schematic illustration of protein fiber fabrication by means of microfluidics. Various types of proteins can be used to spin protein fibers through microfluidic devices.
Recently, microfluidic technology has been investigated and applied as an easy-to-perform method to fabricate biological fibers (Fig. 1) [28–30]. In comparison with other techniques, microfluidics provides a simple platform to mimic a natural spinning apparatus in a controlled manner [31]. For example, Yu et al. [32] developed bio-inspired helical microfibers with controllable conformation by means of a coaxial capillary microfluidic device, which exhibited versatile biomedical engineering applications. Furthermore, the cost-effectiveness of microfluidics makes it an easy strategy for generating continuous microfibers in bulk [33]. The use of biocompatible solvents in fiber spinning has prompted the application of this technology in tissue engineering and drug delivery [34–36]. Moreover, the mass production of protein fibers with diverse morphologies and uniform size can be easily realized by microfluidics. As the channel size and type, flow rate, and shear force can be precisely modulated, mechanically strong protein fibers with controllable morphologies and properties can be facilely prepared via well-designed microfluidic chips.
Herein, we will discuss on recent progress in the fabrication of protein fibers based on microfluidic techniques and their mechanical performance. We will highlight a variety of protein fibers developed via microfluidics including regenerated protein fibers, recombinant protein fibers, and other sources of protein fibers. The construction process and mechanical properties of each type of protein fiber will be discussed, providing a paradigm for the fabrication of artificial protein fibers with technical applications. Finally, we will give a perspective on the development of microfluidic techniques for protein fiber production.
《2. The natural spinning process of spider silk》
2. The natural spinning process of spider silk
The extraordinary mechanical properties of natural spider silk have inspired the fabrication of synthetic fibers over the past decades. Natural spider silks are produced under physiological conditions (aqueous medium, ambient temperature, etc.) by special spinning glands, such as the major ampullate gland, minor ampullate gland, flagelliform gland, pyriform gland, and cylindrical glands [37]. The S-shaped tapered ducts provide an additional shear force for the spinning dope, reinforcing the orientation of protein molecules [38]. In addition, various ions such as H+ and PO43– are secreted by specific cells, promoting the dehydration process and the formation of β-sheet structures. Given their chemico-physical conditions, spider silk fibers have extraordinary mechanical properties (a Young’s modulus of ~15 GPa, strength of ~1.5 GPa, extensibility of ~40%, and toughness of ~200 MJ∙m-3 ). As-spun spider silks are usually three times tougher than Kevlar and five times stronger than steel [39].
《3. The microfluidics spinning process》
3. The microfluidics spinning process
Microfluidics has recently emerged as a very promising approach for producing fibers, in comparison with other spinning technologies [28]. As shown in Table 1, electrospinning usually requires a high voltage and high shear, while melt spinning requires a high temperature [40,41]. In contrast, microfluidics can be implemented under mild conditions with high reproducibility and yield. In addition, the shape, size, and anisotropy of the fibers can be accurately controlled by microfluidics at the micro/ nanoscale level [42]. In general, a microfluidic device consists of a chamber, a reservoir, and channels. To date, different types of channels have been developed, such as the single channel, double channel, and flow-focusing junction [26,27,32]. A ‘‘core–sheath” flow profile is necessary in order to continuously produce fibers, in which the spinning solutions first pass through the channel to generate fibers, followed by the solidification process via solvent extraction, chemical crosslinking, or photolithographic solidification. The as-spun fibers usually have a uniform size in diameter and a smooth surface morphology, which often results in the obtained fibers exhibiting high mechanical performance. As a result, such fibers have potential for application in the biomedical field, such as in drug delivery, bone tissue engineering, and wound closure [9].
《Table 1》
Table 1 Comparison of different spinning techniques.
《4. Regenerated protein fibers by microfluidics》
4. Regenerated protein fibers by microfluidics
Despite the extraordinary properties of natural protein fibers, their mass production is still an unattainable challenge. Therefore, regenerated protein fibers have been considered as promising alternatives that can replicate the hierarchical structures of natural protein fibers. A great deal of effort has been devoted to the development of regenerated protein fibers with high quality and excellent mechanical properties using various approaches, such as WS and electrospinning. However, the developed approaches usually suffer from limited scale, complicated procedures, and the requirement of large amounts of solvents.
To overcome these limitations, microfluidics has attracted considerable attention for fabricating artificial protein fibers, due to its unique features. Recently, Kinahan et al. [43] pioneered the combination of microfluidic techniques with modeling, and consequently achieved prediction and control of the performance of fabricated silkworm fibroin fibers (Fig. 2(a)), with a primary focus on the silk molecular assembly. To emulate the natural silk-spinning process, the microfluidic device with a cross-shaped channel was designed to be controllable, with three inlets and one outlet. In the device, regenerated silk fibroin (RSF) aqueous solution (8% w/v, pH 6.6) flows through the central inlet and reaches the cross intersection; it then interacts with two outer streams of poly(ethylene glycol) (PEG) solution. A customized syringe pump is utilized to control fluid flow, which influences fiber diameter. The post-drawing treatment can also be used to manipulate the fiber diameter. It was found that the post-spinning drawing process (which is carried out on the spun fibers after another spinning process) enhanced the mechanical performance of the as-spun fibers, which had a Young’ modulus of about 3.5 GPa, a tensile strength of about 80 MPa, and a breaking strain of about 16%. The improvement of the fiber’s mechanics, in comparison with undrawn RSF fibers (i.e., just the spun fibers), can be ascribed to the uncoiling region that partially mimics the mechanical performance of natural silk.
《Fig. 2》

Fig. 2. Schematic illustration of RSF fiber fabrication and characterization by microfluidics. (a) (left) The microfluidic device consists of three inlets and one outlet into a reservoir. RSF fibers with smooth surfaces and consistent diameters are produced at the cross intersection. (right) Typical stress–strain curves of the RSF fibers under different conditions. (b) (bottom) A single-channel microfluidic device fabricated to produce RSF fibers. (top left insert) Typical stress–strain curves of the as-spun RSF fibers. RSF: regenerated silk fibroin; SEM: scanning electron microscope; PD-100: post-drawn fiber-100; PD-250: post-drawn fiber-250; AS-250: as-spun fibers 250; PDMS: polydimethylsiloxane. (a) Reproduced from Ref. [43] with permission of American Chemical Society, ©2011; (b) reproduced from Ref. [44] with permission of Elsevier B.V., ©2014.
To mimic the shear and elongation conditions of spider and silkworm silks, RSF fibers were also fabricated from regenerated Bombyx mori (B. mori ) via a single-channel microfluidic chip (Fig. 2(b)) [44,45]. In that study, fibers were produced by extruding the spinning dope (pH ~4.8, 50 wt% RSF solution, and 0.3 mo·lL–1 Ca2+) from the microchip at a flow rate of 2 μL·min-1 . In comparison with degummed silk fibers, the as-spun RSF fiber had a similar diameter (12 μm) but weaker mechanical properties. In stark contrast, the mechanical performance of the as-spun fiber was enhanced significantly by the post-drawing treatment (i.e., an additional drawing step after the fiber is spun), exhibiting excellent mechanical behavior including its Young’s modulus (19 GPa), breaking stress (614 MPa), breaking strain (27%), and breaking energy (101 kJ·kg-1 ), which were superior to those of other reported RSF fibers and to natural undegummed B. mori silkworm silk [43,46].
Subsequently, the microfluidic technique was introduced to fabricate regenerated composite silk fibers. Cellulose nanofibers (CNFs) have shown considerable potential as a reinforcement agent in biocompatible composites due to their biocompatibility and the high stiffness of the crystalline regions [47]. Taking advantage of the mechanical properties and functions of CNFs, Mittal et al. [48] reported the fabrication of composite fibers (90% CNF, and 10% silk), where highly oriented fibers were prepared via a flow-assisted alignment and assembly approach (Fig. 3(a)). In that study, a double flow-focusing device was composed of a core flow of composite dispersions and two surrounding sheath flows. The first sheath flow of deionized water (pH ~5.6) prevented buildup at the channel walls, while the other sheath flow of hydrochloric acid (0.01 mol∙L–1, pH ~2) assisted in the alignment of CNF/silk proteins in the dispersion. In comparison with pristine CNF fibers, the composite CNF/Z-silk fibers had an average modulus of about 55 GPa, a toughness of about 55 MJ∙m-3 , a breaking strength of about 1015 MPa, and an extensibility of about 6%, exceeding those of typical natural or synthetic fibers [39]. In addition, immunoglobulin G (IgG) cell binding tests showed that the composite fibers were biocompatible. All these results indicate that ultra-strong composite protein fibers can be fabricated in a simple, controllable, and industrially scalable way by microfluidics.
《Fig. 3》
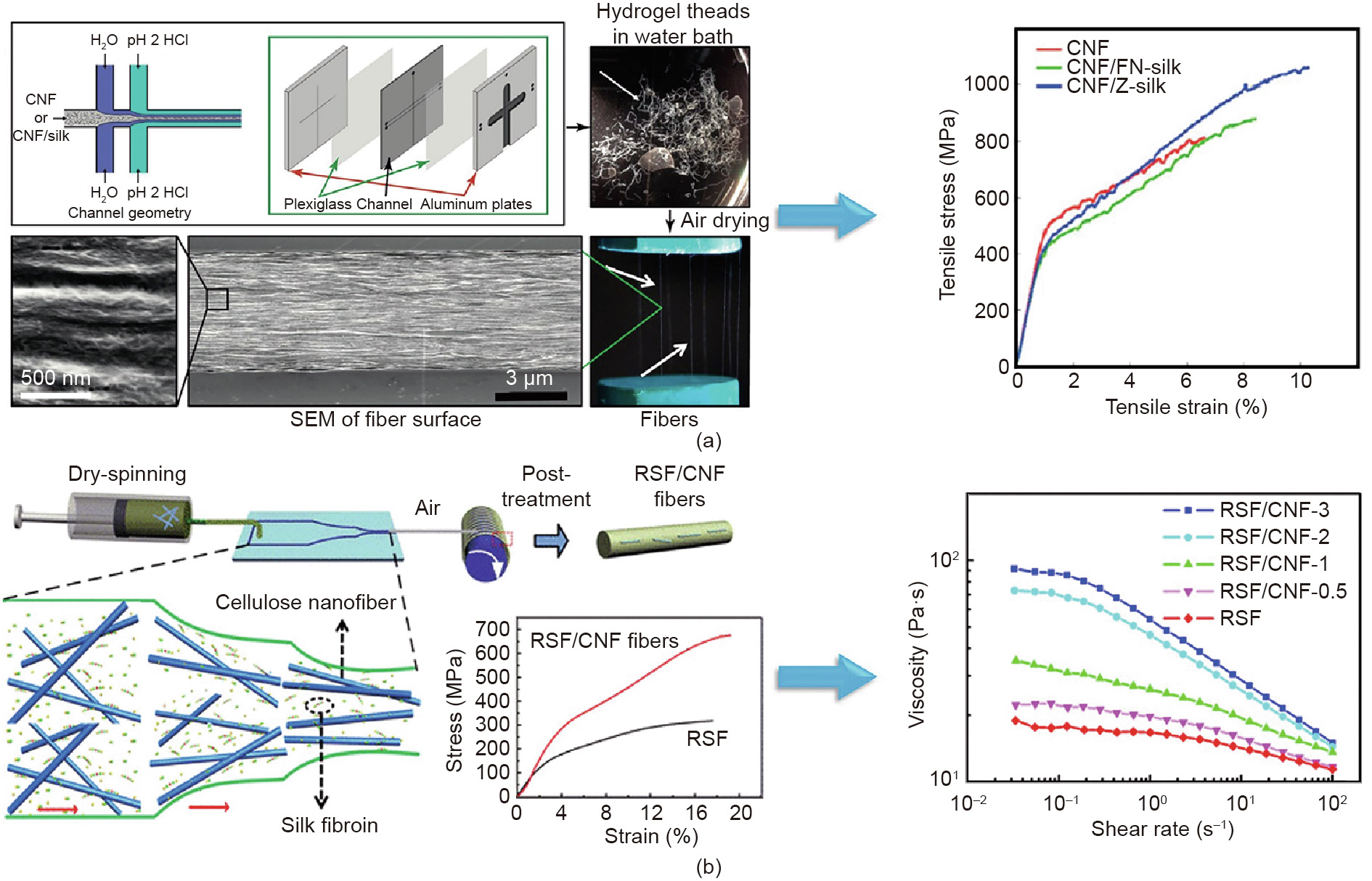
Fig. 3. Microfluidic methods for the fabrication of regenerated composite protein fibers. (a) Schematics of the double flow-focusing device for the generation of composite fibers. The fibers exhibited a significant improvement in mechanical properties with the addition of silk protein. (b) Schematics of the dry-spinning process using a microfluidic device. The mechanical performance of RSF/CNF fibers was significantly increased after the inclusion of 0.1 wt% CNF treatment. (a) Reproduced from Ref. [48] with permission of American Chemical Society, ©2017; (b) reproduced from Ref. [49] with permission of American Chemical Society, ©2019.
Recently, a new type of RSF/CNF hybrid fiber was developed using microfluidics (Fig. 3(b)) [49]. To produce the RSF/CNF hybrid fibers, spinning dope was pumped into a microfluidic channel at a flow rate of 2 μL·min-1 . The as-spun fibers were dried for 48 h and were then immersed in aqueous solution with 80 vol% ethanol. The presence of CNF notably enhanced the mechanical performance of the hybrid fiber. In particular, the breaking strength of the hybrid fibers (containing 0.1 wt% CNF) was increased to 487 MPa, which was significantly stronger than that of pure RSF, and the extensibility was extended to 16%. This behavior could be ascribed to the addition of CNF, which resulted in high crystallinity, a high mesophase content, and a small crystallite size.
《5. Recombinant protein fibers made with microfluidics》
5. Recombinant protein fibers made with microfluidics
In addition to regenerated protein fibers, recombinant protein fibers have been fabricated and investigated with the use of microfluidics. Genetic engineering has been applied to express recombinant proteins that can mimic the hierarchical structures and molecular weight of natural spider fibroins. To date, various recombinant proteins have been used as feedstock to fabricate artificial protein fibers [50–52]. However, the mechanical properties and functions of the resulting fibers are still far from those of natural spider silks [53]. An interesting observation related to spider and silkworm silk glands is that both of these natural spinning mechanisms can be considered to be complex microfluidics. Peng et al. [54] designed biomimetic microfluidic channels to emulate the specific geometry of silkworm silk glands (Fig. 4(a)). In their study, the recombinant major ampullate (MA) spidroin I (MaSp1) of the spider Nephila clavipes (Mw 47 kDa) was expressed in Escherichia coli (E. coli ) and then used as spinning dope in two different spinning processes with a bioinspired microfluidic chip. The first process was microfluidic WS using pure ethanol as a coagulation bath, while the second was modified WS with a post-spin drawing process (WS-PSD). After post-spin drawing treatment, this biomimetic strategy resulted in protein fibers with excellent mechanical properties, which were comparable to some of those reported for recombinant spider dragline silk (Fig. 4(b)).
《Fig. 4》

Fig. 4. Schematic illustration of the fabrication and mechanical properties of recombinant spider dragline silk made using microfluidics. (a) Biomimetic microfluidic devices were designed to emulate the specific geometry of the silkworm silk gland, including WS and WS-PSD. (b) Mechanical performance of recombinant fibers. The typical strain– stress curves of recombinant spider silk fibers of WS-3x (top) and WS-PSD-3x (bottom). Reproduced from Ref. [54] with permission of Springer Nature, ©2016.
《6. Other sources of protein fibers by microfluidics》
6. Other sources of protein fibers by microfluidics
Despite the extensive research that has been performed on biological fibers inspired by spider silk and silkworm proteins, it is still challenging to open up a new way to fabricate robust biological fibers while relying on low-cost proteins and convenient spinning technology. Although other fibrous proteins such as elastin, collagen, and phage virus have been explored for fiber fabrication [55–58], their mechanical properties are still not satisfying.
《6.1. Globular bovine serum albumin-based fibers》
6.1. Globular bovine serum albumin-based fibers
To overcome these limitations, our group recently developed an efficient strategy to fabricate robust protein fibers using globular bovine serum albumin (BSA) with the microfluidic technique (Fig. 5) [59]. A microfluidic device for fiber production was designed with a two-channel glass capillary and a single tapered outlet (300 μm). In the device, BSA solution and glutaraldehyde (GA) solution converge in the capillary and extrude into the 80% (v/v) methanol/water coagulation. The final BSA fibers are collected after the dehydration process. It was found that the mechanical properties of the GA-crosslinked BSA fiber (GBF) exhibited obvious enhancement when compared with those of pristine BSA fiber (PBF). Notably, the post-stretching treatment of the GBFs (P-GBF) further increased the fiber’s mechanics, including its breaking strength (300 MPa), Young’s modulus (4.4 GPa), toughness (50 MJ·m-3 ), and extensibility (30%). Compared with many other protein fibers, the resulting fibers exhibited comparable or even higher mechanical performance.
《Fig. 5》

Fig. 5. Schematics of the fabrication of BSA fibers by a microfluidic method. (a) The device is composed of a two-channel microfluidic chip, a coagulation bath, and a rotation collector. The BSA fibers are spun in the 80% methanol/water coagulation bath, to promote crosslinking by glutaraldehyde (GA) and the dehydration process. (b) Typical strain–stress curves of BSA fibers under different conditions. PBF: pristine BSA fiber; GBF: GA-crosslinked BSA fiber; P-GBF: post-stretching treatment of the BSA fibers. Reproduced from Ref. [59] with permission of Wiley, ©2019.
《6.2. Spherical and linear protein-based fibers》
6.2. Spherical and linear protein-based fibers
A range of proteins with spherical or linear structures, including chicken egg, goose egg, milk, and collagen, have also been investigated for the massive production of biological fibers with microfluidics techniques [33]. In this case, the microfluidic device consisted of two tapered glass capillaries coaxially assembled on a glass slide, featuring interlayer and core layer channels for the protein solution and a 5% GA crosslink agent, respectively. This technique is able to fabricate protein fibers with high tensile strength and toughness, comparable to or even stronger than many reported recombinant spider silks or regenerated silkworm fibers [60–62]. Due to their impressive mechanical strength and biocompatibility, these protein fibers were successfully applied as surgical suturing on rat and minipig models.
With the aim of avoiding the addition of chemical crosslinking agents, Haynl et al. [63] reported the fabrication of single collagen type I microfibers using microfluidics with no crosslinking agent (Fig. 6). In their study, a polydimethylsiloxane (PDMS)-based microfluidic device with tiered channel geometry was designed to enable the circulation of the sheath flow around the protein stream. In the presence of PEG and at an appropriate pH, microfibers were formed at the cross junction and extruded into a water bath, followed by drawing using an automated spool. The results showed that the flow rate can affect the mechanical properties of collagen fibers. A higher flow rate can result in stronger fibers with a tensile strength of (383 ± 85) MPa and a Young’s modulus of (4138 ± 512) MPa, which exceed those of fibers produced with GA crosslinking or 1-ethyl-3-(3-dimethylaminopropyl)carbodii mide (EDC)/N-hydroxysuccinimide (NHS) crosslinking [55,64]. Furthermore, the axon growth of neuronal cell NG108-15 along the microfiber axes revealed the potential application of these fibers in peripheral nerve repair [42].
《Fig. 6》

Fig. 6. Schematics of the fabrication and mechanical properties of collagen and whey protein isolate (WPI) fibers by means of microfluidics. (a) (top) A microfluidic chip is connected to three independent syringe pumps that allow simultaneous pumping of a collagen solution at pH 3 (red) and two PEG-containing buffer solutions at pH 8 (blue). The microfibers are extruded into a water bath and drawn by an automated spool. (bottom) Typical strain–stress curve of collagen fibers. (b) (top) Schematics of the double flow-focusing device for fiber assembly. The PNF dispersion, deionized water, and acetate buffer (pH 5.2) are injected in the core flow, first sheath flow, and second sheath flow, respectively. (bottom) The typical stress–strain curves reveal a rather brittle property, with a Young’s modulus of about 288 MPa and an extensibility of about 1.5%. (a) Reproduced from Ref. [63] with permission of American Chemical Society, ©2016; (b) reproduced from Ref. [65] with permission of National Academy of Sciences, ©2017.
《6.3. Whey protein isolate-based fibers》
6.3. Whey protein isolate-based fibers
More recently, whey protein isolate (WPI) has been explored as an alternative platform for constructing protein fibers. Kamada et al. [65] reported a bottom-up assembly strategy to fabricate microfibers by means of microfluidics (Fig. 6(b)). In their study, a double flow-focusing microfluidic device was used to produce amyloid-like protein nanofibrils (PNFs) at a concentration of 0.45%–1.8% (w/v) with a pH of 5.2. Interestingly, the curved PNFs with a lower degree of fibril alignment resulted in mechanically strong microfibers with a Young’s modulus of approximately 288 MPa and an extensibility of about 1.5%. Kamada et al. [66] designed a flow-focusing microfluidic device to fabricate hierarchical protein macro-sized fibers from β-lactoglobulin, which can self-assemble into nanofibrils. The b-lactoglobulin was utilized to prepare a spinning dope solution to co-flow with a CaCl2 cross-linker solution at the junction. Macroscopic fibers were fabricated by adding PEG to the sheath flow and then immediately collected from the outlet of the channel. The diameter and mechanical properties of the as-spun fibers could be precisely controlled by manipulating the sheath flow rate. In particular, the Young’s modulus and tensile strength of the as-spun fiber were further improved to (2.21 ± 0.4) GPa and (92.0 ± 28.0) MPa, respectively, by the addition of the preformed nanofibrils and their alignment.
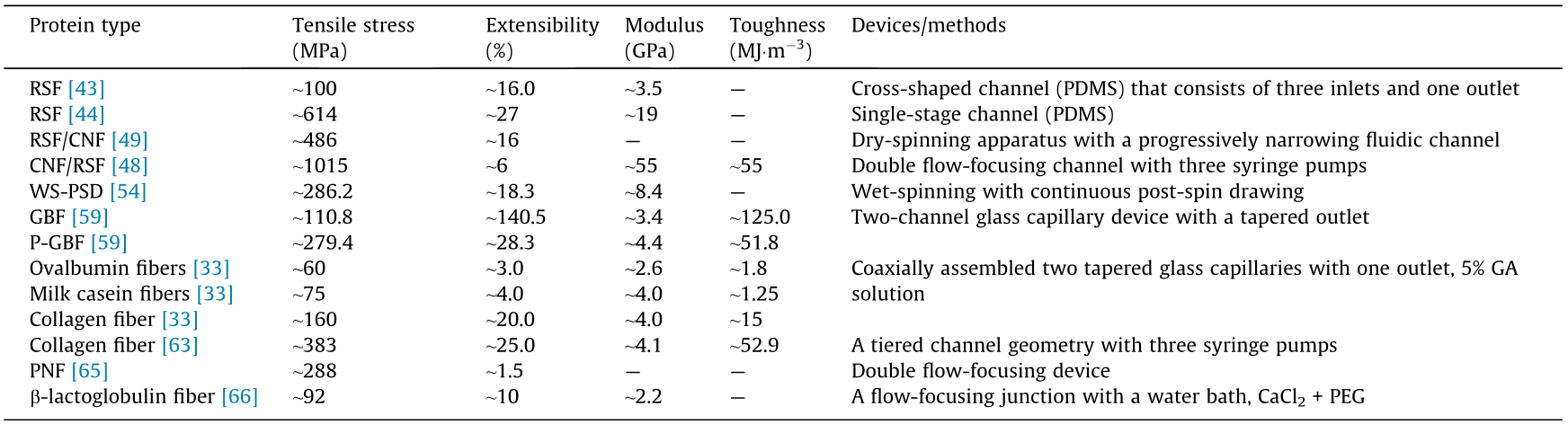
Table 2 summarizes a comparison of the mechanical performance of different types of protein-based fibers developed by means of microfluidics. This comparison indicates that microfluidics can provide a powerful platform for the fabrication of protein fibers with admirable mechanical performance. Such a universal strategy not only works for regenerated silk proteins and recombinant proteins, but is also suitable for other commonly used proteins. In particular, proteins that are widely available in nature, such as egg ovalbumin and milk casein, can be used to fabricate robust protein fibers by means of microfluidics. In addition, the protein fibers produced by microfluidics exhibit good biocompatibility when compared with those fabricated using cytotoxic crosslinking agents. Moreover, the microfluidic setup offers excellent potential for the fabrication of protein fibers with a rather low concentration, resulting in as-spun fibers with a more distinct alignment. However, the mechanical performance of the as-spun fibers produced using microfluidics is still inferior to that of natural spider silk fibers. Thus, more efforts are required to investigate the relationships among fiber mechanics, channel size and types, flow rates, shear force, and hierarchical protein structures.
《Table 2》
Table 2 Comparison of the protein fibers produced by microfluidic devices.
《7. Conclusions and outlook》
7. Conclusions and outlook
Eco-friendly and robust protein fibers are rapidly emerging materials that have been widely used in the field of tissue engineering, in addition to some high-tech applications. Recently, significant advances have been achieved in the fabrication of protein fibers by means of microfluidics. Compared with other spinning techniques, the microfluidic method offers convenient and controllable operation at the microscale to produce continuous protein fibers. In addition, this method has exceptional advantages including low cost, easy fabrication with well-designed structures, and simple post-processing. Moreover, the flexible incorporation of varied functional materials in the fibers produced using microfluidics offers further opportunities to enhance the mechanical performance of the as-spun fibers. Given the remarkable advantages of this technology, microfluidics can realize the fabrication of mechanically strong fiber materials with multiple functions on a large scale.
Although mechanically strong protein fibers can be generated in a well-designed microfluidic device by precisely controlling the channel size and type, flow rate, and shear force, there are still some deficiencies in the fiber processing. It is well known that protein structure plays a pivotal role in determining the mechanical behaviors of protein-based fibers. However, microfluidic chips are insufficient to effectively control protein structures at the nanometer scale so far. This method also lacks effective strategies for the construction of protein fibers with hierarchical structures, such as core–shell, multi-strand, spiral, or Janus-type structures. In addition, the integration of monofilament and twisting spinning technology in a single microfluidic chip is still problematic. Furthermore, it remains challenging to design multiple channels to generate complex protein fibers.
The design of a new class of microfluidics that does not sacrifice the structure and orientation of proteins is highly desirable for improving the mechanical behaviors and functionalities of the as-spun fibers. To further promote the application of microfluidics in the fabrication of protein-based fibers, new manufacturing techniques such as three-dimensional or four-dimensional printing could be integrated into the design of microfluidic chips. Designing microfluidics with unique stimuli-responsive features could also facilitate the control of the as-spun fiber properties. The development of advanced microfluidics in the fabrication of protein-based fibers with various tailored properties, compositions, structures, and on-demand functionalities will pave the way for practical applications for biological fibers.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Jing Sun, Jingsi Chen, Kai Liu, and Hongbo Zeng declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




