《1. Introduction》
1. Introduction
Carbohydrates are one of the most widely used substances in nature and one of the main ingredients of food. Dietary carbohydrates dominate the energy sources of the human diet and are the main determinants of postprandial blood glucose levels (Fig. 1). Besides carbohydrates, carbohydrate-rich foods are also largely influenced by the amount and type of dietary fiber as well as the physical structure of the corresponding starch. In addition, these foods can modulate the blood glucose response in vivo. The concept of the glycemic index (GI) was firstly introduced in 1981 to deal with these factors [1]. The GI quantifies the potency of carbohydrate foods to raise blood glucose levels. Further, it can provide a quantitative description of the rates of glucose clearance in vivo. In addition to the GI, the concept of ‘‘glycemic load” (GL) was recently introduced to represent the product of the GI of a food and the amount of available carbohydrates present in that food. The main advantage of the GL is its ability to quantify the overall glycemic effect of carbohydrate-rich foods [2].
《Fig. 1》
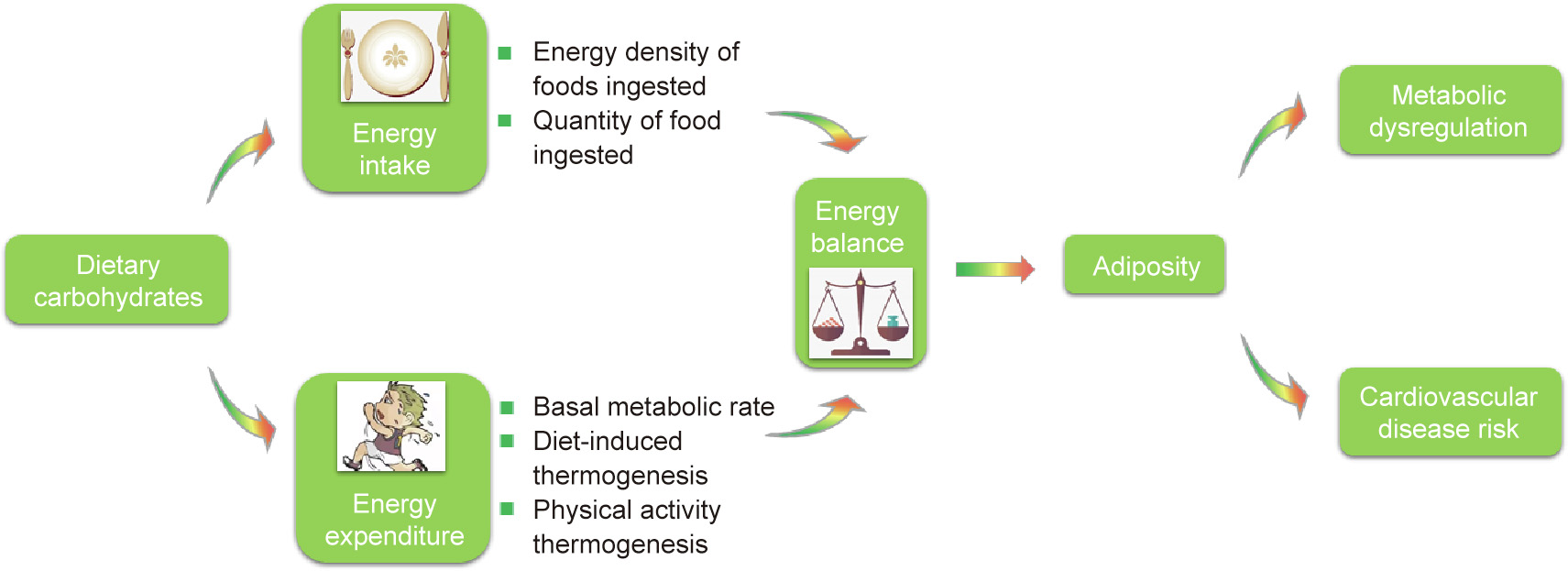
Fig. 1. Schematic illustration of the interaction between the individual components of energy balance and dietary carbohydrates, together with the influence of culmination on human health. Reproduced from Ref. [3] with permission of Oxford University Press, ©2017.
Carbohydrates constitute a large portion of the caloric intake in Western diets, and literature reports have revealed that it specifically constitutes almost half of that caloric intake; 22%–40% of modern-day hunter-gatherer diets comprise carbohydrates [4]. Owing to the diversity of carbohydrate-rich foods, our nutritional status and health are fully satisfied. Moreover, these foods increase our pleasure and enjoyment of food. However, in addition to the favorable characteristics of carbohydrate-rich foods, there are various concerns related to their possible side effects. For instance, these foods have been reported to have some unwanted effects on glycemic control and plasma lipid concentrations. More critically, reports have shown that a high intake of refined carbohydrate foods increases plasma glucose and insulin levels during the postprandial period. Moreover, this can also contribute to increase postprandial plasma triglyceride levels, elevate fasting, and reduce high density lipoprotein (HDL) cholesterol levels [5].
Carbohydrates can be classified into three different types: sugar, starch, and fiber (non-starch polysaccharides). Sugar has played an important role in human history, and there are numerous records of the essential roles of sugars in dietary patterns. This mainly results from the foundational role of sugars in biological life. For example, glucose, despite its simple structure, is one of the essential medicines of the World Health Organization (WHO) owing to its central role in human health. Although sugars are important energy suppliers in the human body, the intake of high doses of sugar is a metabolic challenge and can cause certain diseases [6]. The intake of doses of sugar (including total, added, and free sugars) above the recommended levels is presently a global public health concern. As a result, the WHO has reviewed its guidelines on sugar intake; they recommend reducing the free sugar intake in adults and children to less than 10% of the total energy intake [7,8]. Free sugars include monosaccharides and disaccharides, which are added to foods by manufacturers, cooks, or consumers, as well as sugars naturally present in honey, syrups, juices, and concentrated juices [8]. Despite their unfavorable effects on human health, new saccharides, which are functional oligosaccharides intermediate between simple sugars and polysaccharides (Fig. 2), could benefit human health. This is mostly attributed to their important physicochemical and physiological properties. As a result, there is a great demand to increase the amount of oligosaccharides in diet foods [9]. Compared with simple sugars, functional oligosaccharides have unique advantages. For instance, functional oligosaccharides do not stimulate an increase in blood glucose or insulin secretion because of the slight release of glucose. Moreover, these can modify the intestinal microbiota and improve the intestinal environment [10,11]. The suppression of diarrhea and the symptoms of diarrhea can also be achieved through the intake of functional oligosaccharides. In addition, the adsorption efficiency of various minerals, including calcium, magnesium, and iron, can be increased [12,13]. Lastly, a reduction in ‘‘civilization diseases” related with lifestyle, such as colon cancer and obesity, can also be achieved. Hence, the consumption of functional oligosaccharides reduces the above risk [14] (Fig. 3).
《Fig. 2》
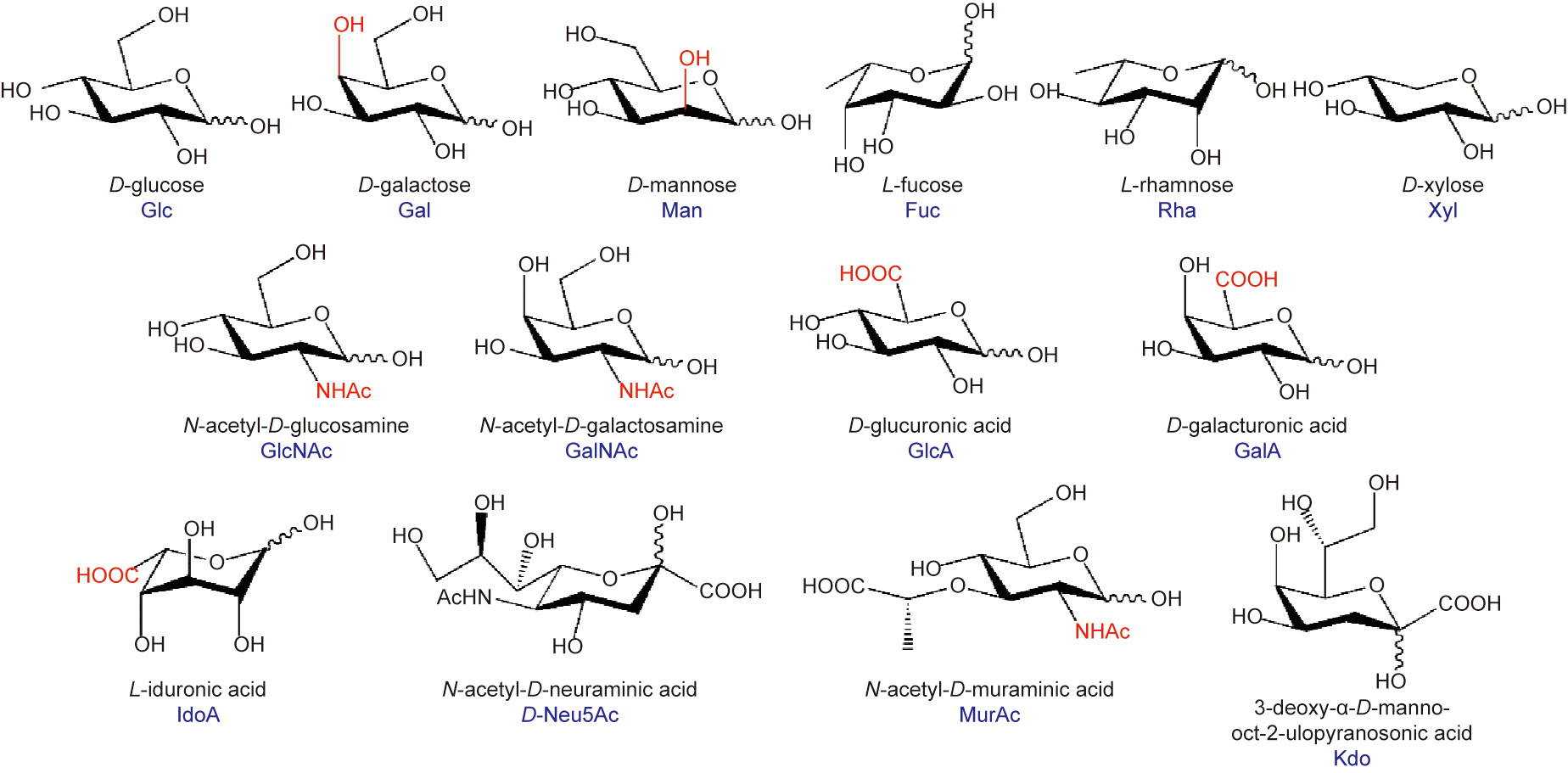
Fig. 2. Common monosaccharide components of functional oligosaccharides. Reproduced from Ref. [15] with permission of Elsevier B.V., ©2017.
《Fig. 3》
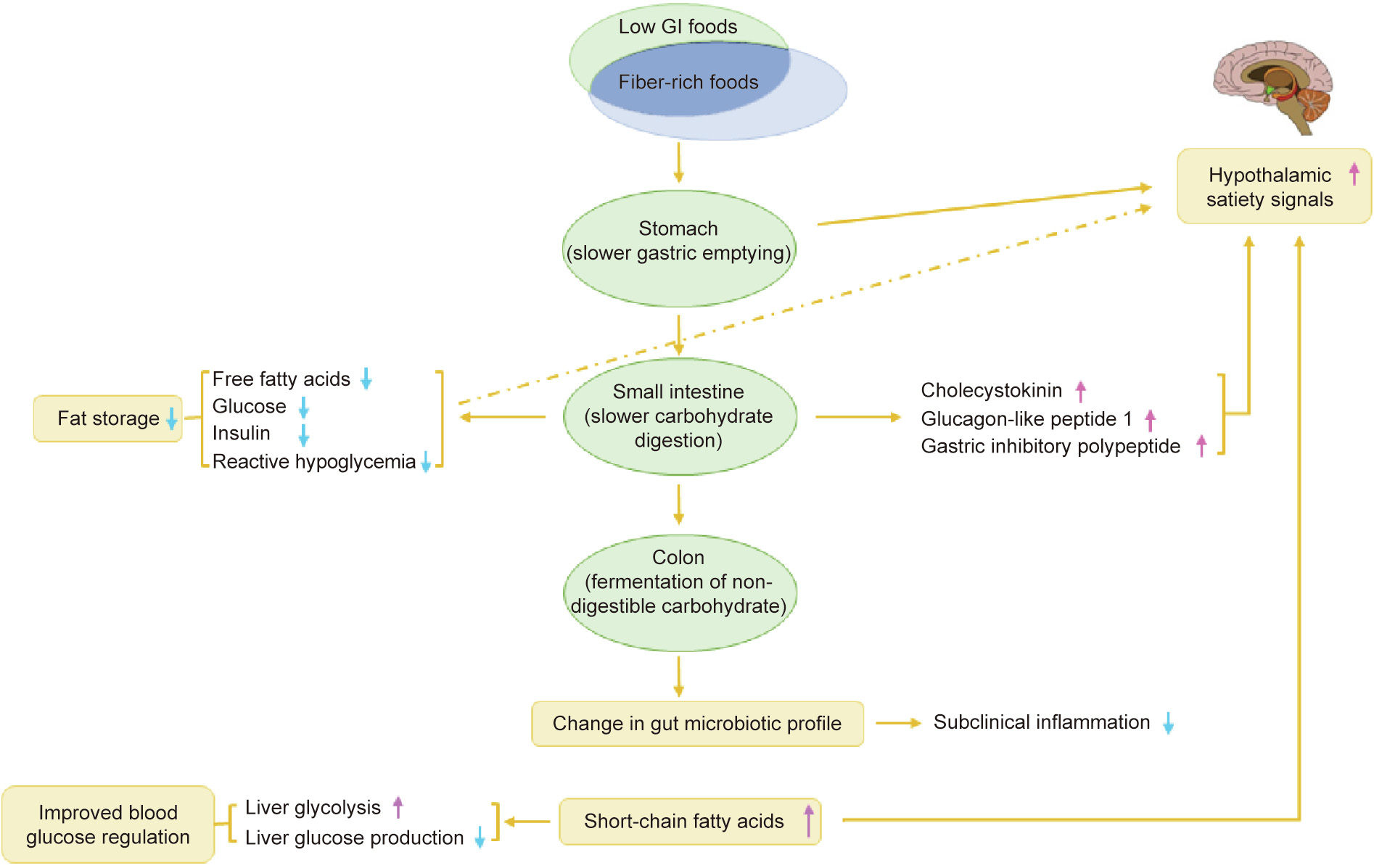
Fig. 3. Summary of possible mechanisms of low GI and fiber-rich foods. Reproduced from Ref. [16] with permission of Springer Nature, ©2012.
As one of the most abundant reserves of carbohydrates, starch is widely found in different plant organs, such as tubers, roots, leaves, fruits, and seeds. In the human diet, the grains of rice, wheat, and maize or the tubers of potato and cassava are staple foodstuffs and a major source of energy [17]. Dietary starch is actually a glucan or, more specifically, a chain of D-glucose monomers linked via glycosidic bonds; once digested by intestinal amylases, it can liberate glucose, which is easily absorbed by the human body [18]. Foodprocessing industries and consumers require starches to have behavioral characteristics that are better than those of native starches. Fortunately, the shortcomings of native starches can be overcome by chemical, physical, or enzymatic modification. Functional starch is starch that has been modified to have specific functional properties, such as physiological functions, health benefits, absorption functions, sustained slow energy release, and other functions endowed via different modification methods. There has been intense interest among researchers to develop novel methods for the preparation of functional starch, with greater emphasis on enzymatic and physical modifications. The multiscale structures and physicochemical properties of functional starch have been well-determined using modern analytical techniques.
There has been a growing interest, on the part of modern consumers, in their personal health: The critical requirements for dietary foods are that the foods should not only be tasty and attractive but also safe and healthy. As a result, nutritionally designed foods are preferred. There are various physiological functions of nondigestible carbohydrates, including dietary fiber, oligosaccharides, and resistant starch (RS), that have garnered increasing attention.
《2. Classification, preparation, and application of dietary starch》
2. Classification, preparation, and application of dietary starch
As a primary dietary source of energy, starch has a biopolymer function and acts as a reserve carbohydrate in many plant organs. After a meal, the digestion rate of starch in the small intestine increases, raising the blood glucose and insulin levels; this correlates highly with nutrition. As a reduction in the glycemic response is considered healthy, Englyst et al. [19] classified starch into three different fractions—rapidly digesting starch (RDS), slowly digesting starch (SDS), and RS; this classification is based on the corresponding digestion rate. In this section, the three dietary starches and their influence on the human body are discussed in detail. In addition, methods for the determination of starch-based foods are explained.
《2.1. Rapidly digesting starch》
2.1. Rapidly digesting starch
2.1.1. Definition and physiological properties of RDS
RDS constitutes a major proportion of dietary starch. High levels of RDS are found in starchy foods cooked using moist heat (bread and potatoes). RDS is defined as a type of starch that is rapidly (within 20 min) converted to glucose molecules via enzymatic digestion [19]. RDS is significantly correlated with the GI based on the in vivo postprandial glycemic response [20]. If RDS is present in high proportions in food, it will rapidly release glucose into the blood and thereby elevate the blood glucose and insulin levels; this is detrimental to health [21].
2.1.2. Preparation and application of RDS
The digestibility of starch is greatly affected by the foodprocessing conditions [22,23], which further affect the proportion of RDS in food. Generally, the proportion of RDS increases after gelatinization. Reports have shown that the content of RDS in waxy rice starch increases after gelatinization [24]. High-temperature ripening also increases the amount of RDS in many types of cereal starch. Grains cooked using a microwave oven, rice cooker, conventional oven, and pressure cooker contain a higher proportion of RDS [25,26].
《2.2. Slowly digesting starch 》
2.2. Slowly digesting starch
2.2.1. Definition and physiological properties of SDS
SDS refers to a starch fraction with a slow digestion rate in the small intestine. The in vitro Englyst assay presents a digestion rate of 20–120 min for SDS [23,27]. Owing to this low digestion rate, SDS is considered by the Institute of Medicine (USA) as a kind of dietary fiber, with reference to the indigestible or less digestible carbohydrates contained in plants [28]. With regard to the health effects of SDS in the human body, SDS possesses the ability to ensure stable postprandial glucose metabolism and reduce the risk of diabetes; moreover, it ensures better mental and physiological performance.
Moreover, compared to RDS with its high GI, SDS has a medium to low GI, leading to a reduction in the GL of the food product [29]. Compared to meals containing rapidly cooked and digestible corn starch, those containing slowly digestible waxy corn starch result in lower peak concentrations of plasma glucose and insulin [30,31]. Compared with RDS, these results suggest that the consumption of SDS could cause blunted postprandial glucose and insulin responses. Furthermore, this would potentially result in a steadier supply and release of energy over time. Based on previously reported literature, the intake of slowly available glucose in obesity could lead to an improvement in the metabolic profile. This situation occurs particularly in postprandial insulinemia along with circulating triacylglycerols and apolipoproteins in triacylglycerol-rich lipoproteins at a lower level [32]. Generally, owing to the excessive dietary intake of carbohydrates and lipids, diabetes is considered a type of metabolic disorder. Further, owing to the absolute or relative lack of insulin in the blood, type 2 diabetes mellitus (T2DM) has emerged as a common endocrine and metabolic condition. Literature reports have shown that foods containing SDS could cause an improvement in the carbohydrate metabolism and facilitate a concomitant reduction in the insulin requirements of insulin-treated T2DM patients [33].
In a test conducted on healthy volunteers, compared to rapidly available carbohydrates, a breakfast that mainly contained SDS resulted in a decrease in performance in the morning [34]. Glucose provides energy for the brain, and studies on glucose drinks have also revealed a positive effect: Glucose tended to improve attentional processes by 8% (P < 0.07) [35]. This suggests that a stable and low insulin response after the intake of a meal plays an important role in satiety regulation, which further supports the beneficial effects of SDS on satiety. Investigations that compared slowly digesting barley kernels with white bread controls showed similar results [36]. Thus, it can be concluded that SDS has an impact on satiety-influencing factors, that is, post-prandial blood glucose and insulin levels. However, the additional mechanisms underlying satiety should continue to be studied, including the characteristics of adsorption, the composition of a meal, gut hormones, and contact with the small intestine.
2.2.2. Preparation and application of SDS
To date, SDS is mainly produced via enzymatic, physical, and chemical approaches. Enzyme-debranching treatment generally uses α-amylase, pullulanase, or isoamylase [37], leading to the hydrolysis of the α-1,4- and α-1,6-glycosidic bonds in starch molecules, thereby destroying the structure of starch and producing shorter chains. The pullulanase debranching-recrystallization method was applied to gelatinize waxy corn starch to prepare high-content SDS [38]. The results indicated that enzymatic debranching treatment was favorable for producing SDS. In terms of chemical modifications, esterification with octenyl succinic anhydride (OSA) is one of the most effective modifications for increasing SDS. During human trials, a marked low glycemic response induced by OSA-modified starch was demonstrated, which is consistent with the extended glucose-release profile of SDS [39]. Ordinary starch (amylose 27%), waxy corn, horny waxy corn (amylose 0%), and high amylose (amylose 50%) were modified via propylene oxide crosslinking or dextrinization to prepare SDS [40]. The study reported that an adjustment of 50% could be achieved for the moisture content of sweet potato starch; after heat treatment at 55 °C for 12 h, SDS with a maximum content of 31% was formed [41]. In addition, the moisture of waxy rice starch and non-waxy rice starch was adjusted to 20% (wet basis), and SDS with better thermal stability was obtained after heating in a microwave for 1 h at the melting temperature of crystallized starch [42].
SDS can be added to various food products, such as cakes, bread, cookies, pizza, dietary supplements, and diabetes-related products, and can help control the release of energy. In addition, SDS products, which can prolong the release of glucose, may provide athletes with enough energy to avoid the exhaustion associated with endurance sports activities. Calorie-associated health hazards can also be avoided by replacing the fat in typical foods with carbohydrates [43]. Chickens have the ability to increase the secretion of pancreatic amylase via an increase in the ingestion of starch [44]. The capacity binding and entrapping of a wide range of hydrophilic and hydrophobic compounds can be strengthened via interactions between functional groups from the starch matrix and the compounds; this could make corresponding derivatives ideal candidates for application in the field of drug delivery.
《2.3. Resistant starch 》
2.3. Resistant starch
2.3.1. Definition and physiological properties of RS
Unlike the other two starches, RS cannot be digested in the small intestine and can only be fermented in the colon. Starch fractions that can be digested within 20 min of incubation are referred to as RDS (determined using the in vitro Englyst assay). A digestion time between 20 and 120 min is a characteristic of SDS, and RS is the remaining fraction without a digestion ability [23]. RS is a mixture of numerous different types of materials, which can be classified into five categories: physically inaccessible starch (RS1), ungelatinized starch granules (RS2), retrograded starch (RS3), chemically modified starch (RS4), and starch–lipid complex (RS5) [45–47]. RS has been found to have beneficial effects on human metabolism and colonic health—improving glycemic and insulin responses, controlling lipid metabolism, modulating gut microbiota-like probiotics, and preventing colonic cancer. In this section, the benefits of RS on human health are introduced from the aspects of gut health, hypoglycemic/hypocholesterolemia effects, and the inhibition of fat accumulation as well as bile stone formation.
Although RS cannot be digested in the small intestine, it can be digested in the large intestine owing to the fermentation caused by probiotic bacteria; this can lead to the release of short-chain fatty acids (SCFA), organic acids, and alcohols. Among these, butyrate has a special effect that causes a reduction in the risk of colon cancer; moreover, inhibition of cancer cell proliferation and stimulation of apoptosis can be achieved through butyrate treatment of cultured colon cancer cells [48]. Reportedly, RS has the potential to counteract the risk by inducing greater fiber fermentation in the distal colon and attenuating red meat-induced colorectal DNA lesions [49].
As non-digestible carbohydrates, prebiotics beneficially influence the host through the selective stimulation of the growth and/or activity of probiotics in the gastrointestinal tract. As it is not absorbed in the small intestine, RS promotes the growth of specific advantageous microbiota by supplying energy to them [50]. For example, RS results in the growth of bifidobacteria in the gastrointestinal tract of rats [51] and the enhancement of the survival of bifidobacteria in acidic buffer or bile acid solutions [52]. Experimental investigations confirmed the favorable interactions between probiotic bacteria and RS through a time-dependent shift in fecal and large bowel SCFA profiles [53].
Extensive research related to the hypoglycemic/hypocholesterolemia effects of RS has been conducted. Compared with other carbohydrates in healthy subjects, the consumption of RS could lower the glucose and insulin levels in serum [54]. Reportedly, RSrich foods can lead to reductions in postprandial blood glucose levels in humans [55]. This suggests that these foods might play an important role in metabolic control supplementation in T2DM. Research focused on the effect of RS3 on cholesterol metabolism in normal and hypercholestrolemic rats has shown [56] that RS has no influence on cholesterol metabolism in normocholestrolemic rats. However, it could lead to substantially lower free and total cholesterol concentrations in the plasma of hypercholestrolemic rats. Meanwhile, a study focused on the effects of RS and cellulose on blood and liver lipids in hamsters have shown that RS could lead to a reduction of 16.2% in the serum cholesterol levels whereas cellulose could lead to a reduction of 13.5% [57]. Despite these numerous investigations, the effects of RS on the total cholesterol level in humans continue to be debated. Therefore, more research is required to understand the influence of RS on cholesterol metabolism in humans.
The reduction in the epididymal and retroperitoneal fat induced by RS-rich foods could lead to significant changes in body fat distribution [58]. Moreover, dermatan sulfate significantly influences bile stone formation through the increased secretion of insulin; this in turn results in the stimulation of the synthesis of cholesterol. As a result, RS causes a reduction in the incidence of bile stone formation [59]. As the milling process increases the digestibility of starch, rice and wheat are the main sources of digestible starch [60]. The intake of RS is 2–4-fold higher in developed countries such as the United States, Europe, and Australia than in populations that consume high starch foods in developing countries such as India and China [46]; further, this might explain the greater number of gallstone cases in the former countries [61,62].
2.3.2. Preparation and applications of RS
Multiple modifications have been applied for the preparation of RS. Researchers investigated the influence of hot steam on RS formation and found that the treatment of legume starch with hot steam increased the content of RS [63]. However, a slightly longer treatment time leads to the hydrolysis of starch and caramelization Moreover, the high cost of steam treatment limits its promotion and application. Single-screw extrude was used to prepare RS using mango starch and determine the change in the RS content before and after starch emulsion extrusion [64]. Researchers developed a heat-stable hypoglycemic starch by gelatinizing common corn starch and subjecting it to autoclaving and enzymatic hydrolysis treatment [65]. Further, reportedly, potato starch can be greatly improved via the use of pullulanase debranching treatment before hot–cooling cycle treatment [66]. In another study [38], after the gelatinization of waxy corn starch via pullulanase debranching, the RS quality score reached up to 50.1% [67]. After the debranching treatment of starch molecules using pullulanase, the probability of molecular chain collision and aggregation as well as the formation of a dense crystal structure greatly increased, and the yield of RS3 increased. Two distinct tuber starches from cassava and potato modified via a chemical reaction OSA were compared with regard to the formation of RS4. In addition, normal corn starch modified using OSA and high-amylose corn starch were assessed in terms of their enzymatic hydrolysis rates [68].
RS possesses unique physicochemical benefits, including gel formation, fine particle size, bland flavor, whitish color, swelling index, increased viscosity, and water-holding capacity. Therefore, RS is widely utilized in numerous foods, such as cakes and brownies. RS3 is routinely used in fried battered foods because of its ability of withstand high temperatures. Further, RS can improve the sensory features of baked or fried foods, such as color, flavor, oiliness, and crispness.
《3. Determination of the digestibility of starch-based dietary food》
3. Determination of the digestibility of starch-based dietary food
The duration and the rate of a glycemic response are usually used to represent the digestibility of starch. The in vivo and in vitro digestion curves of starch are shown in Fig. 4. There are several methods used for the accurate determination of the digestibility of starch-based dietary food to monitor the rate and extent of starch digestion as well as the intestinal absorption of starch-derived glucose. This allows manufacturers to alter the glucose response of each food, especially in the case of diabetic therapeutic foods, diabetes management, and carbohydrate metabolism disorders. In this section, the main determination method and the index of the digestibility of starch-based dietary foods are introduced in detail.
《Fig. 4》
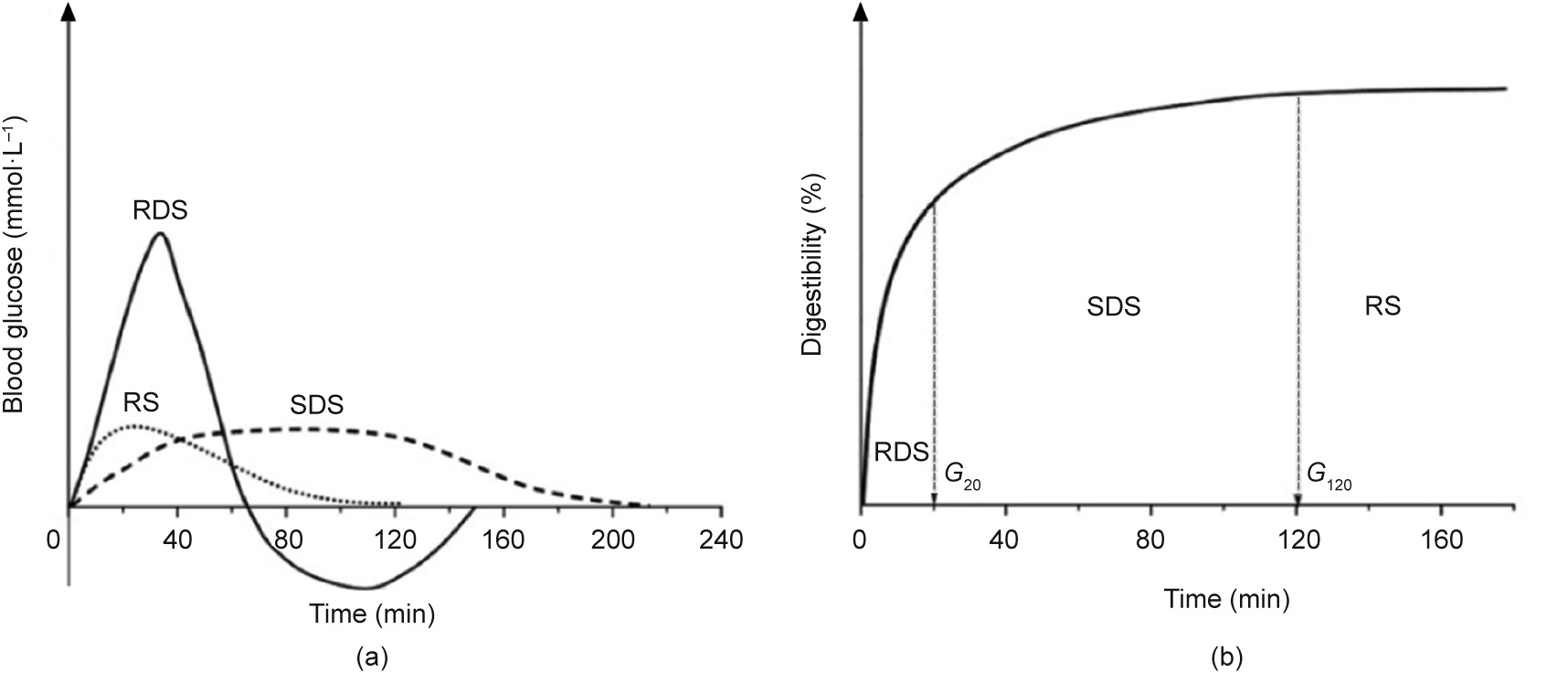
Fig. 4. The bioavailability of nutritional starch fractions. (a) Glycemic response to RDS, SDS, and RS in vivo; (b) digestion assay in vitro using Englyst method. G20 and G120 represent the amounts of glucose after digestion for 20 and 120 min, respectively. Reproduced from Ref. [23] with permission of Taylor & Francis, ©2015.
《3.1. In vivo starch digestion 》
3.1. In vivo starch digestion
Starch is acted upon by salivary amylase in the mouth, which is the first enzyme to act on carbohydrates during digestion [69]. In a relatively short time, the bolus of food is carried by esophageal peristalsis into the stomach, which secretes HCl. The pH of gastric juice is roughly 2.6, retarding the action of α-amylase but increasing the acid hydrolysis of starch. From the stomach, the ingested starch proceeds to the duodenum. Pancreatic fluid contains two important components for starch digestion: bicarbonate and α-amylase. The former neutralizes the acidity of the fluid arriving from the stomach to pH 8. In addition, α-amylase continues to hydrolyze starch into glucose and oligosaccharides. The obtained oligosaccharides, which contain linear and branched structures, may not be absorbed into the bloodstream without further hydrolysis into glucose; therefore, further enzymatic processes are required [70].
Substrates not digested by α-amylase are degraded into single glucose units by mucosal maltase–glucoamylase and sucrase–isomaltase in the small intestine. These are exo-glucosidases that catalyze α-1,4 bonds and α-1,6 branch bonds, ensuring the further degradation of non-linear oligosaccharides. The obtained monosaccharides are absorbed into the bloodstream via secondary active transport across the apical membrane of the enterocytes and subsequently exit the gastrointestinal tract across the basolateral membrane [71]. Undigested polysaccharides, such as RS and soluble fiber, are digested through fermentation in the large intestine (colon), which contains a very large population of microorganisms. Fermentation leads to the formation of SCFA, which stabilize blood glucose levels and suppress cholesterol synthesis in the liver [72,73].
The GI refers to the incremental area under the glucose response curve of the 50 g effective carbohydrate portion of a test food (the changes in the blood glucose levels at 120 min after meals); it is expressed as a percentage of the response to the same amount of carbohydrates of a standard food (white bread or glucose) in the same subject [74,75]. Data of the hydrolysis of novel starch products in vivo can be used effectively to predict glycemic responses in vivo. Plasma glucose responses are largely assumed to be associated with insulin secretion. It has been suggested that the insulin index (II) can be used for the dietary management of patients with diabetes. The equation for calculating II is similar to that for calculating GI, which was developed by Jenkins Granfeldt et al [76]. Furthermore, a significant correlation between hydrolysis index and II was found using the in vivo method [76].
《3.2. In vitro starch digestion》
3.2. In vitro starch digestion
Englyst et al. [19] developed the most widely accepted method to quantitively estimate nutritional starch fractions from complex food materials to pure starches; this method involves the stimulation of the human digestive system. Controlled enzymatic hydrolysis with pancreatic amylase and amyloglucosidase was used to determine various kinds of starch constituents. Glucose oxidase was used to measure the released glucose. The equation for the calculation, which is based on Englyst’s method, is shown as follows:

where GFG, GTG, and STS represent the amount of free glucose, total glucose, and total starch, respectively; G20 and G120 represent the amounts of glucose after digestion for 20 and 120 min, respectively; SRDS, SSDS, and SRS represent the amounts of RDS, SDS, and RS, respectively; and 0.9 represents the conversion factor from glucose to starch considering the re-movement of one water molecule per glucose unit. Moreover, numerous attempts have been made to modify these equations. For instance, the Netherlands Organization for Applied Scientific Research (TNO) has made two major improvements: a mixture of microbial enzymes and the analysis of the amount of glucose released at specific time points. Published literature has shown that the GI values obtained using the TNO method are consistent with the results obtained from human studies comparing various pure carbohydrates with different kinds of carbohydrate-containing food products [77]. Admittedly, Englyst’s method still has certain drawbacks, including the troublesome demand for a wide variety of substrates, the lengthy procedure, and its poor reproducibility.
In addition, factors such as starch characteristics and the composition of starch-based foods could influence the process of in vitro starch hydrolysis. A wide variation in the hydrolysis rate of starch exists among different food products: Lentils have the lowest rate, whereas boiled potatoes have the highest. Goñi et al [78] proposed an improved in vitro method to investigate the hydrolysis of starch at different time intervals. A first-order equation was proposed for the hydrolytic process of starch in foods as shown below:

where C represents the concentration of starch hydrolyzed at time t, 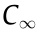 is a constant representing the equilibrium concentration, and k represents the kinetic constant.
is a constant representing the equilibrium concentration, and k represents the kinetic constant.
Numerous researchers have confirmed that changes in starch viscosity influence digestibility. The extent of the dissolution of starch granules and the subsequent enzymatic hydrolysis can be characterized using a commercially available controlled stress rheometer [79]. Research has revealed that a decrease in the viscosity occurs over the course of a simulated digestion; moreover, the extent to which the viscosity of partially digested intestinal contents is increased can be predicted using the relative viscosity [80]. Further, the metabolic behavior of starchy foods can also be predicted. A method for measuring the digestion rate of in vitro starch in products has been introduced to predict such behavior based on a peculiar ‘‘as eaten” (chewing/dialysis test) structure [76].
《4. Architecture of novel starch-based dietary foods 》
4. Architecture of novel starch-based dietary foods
《4.1. Design of low-GI foods through the direct addition of modified starch》
4.1. Design of low-GI foods through the direct addition of modified starch
With the improvement of living standards, the incidence of diet-associated chronic metabolic disorders has been increasing year over year [81]. The GI of foods has gained increasing attention, and relevant research has expanded. Studies have shown that the long-term intake of high-GI foods correlates closely with the occurrence of obesity, hyperlipidemia, diabetes, and hypertension as well as cardiovascular and cerebrovascular diseases. GI has great guiding value and strong operability in preventing and controlling certain chronic diseases [82–84].
Further research has shown that, with the currently used methods for preparing low-GI foods, which feature low-GI raw materials, such as modified starch and recombined nutritional products prepared using extrusion technology, the ultimate purpose is to improve the product content of RS and SDS. GI does not increase rapidly over a short period of time.
Researchers have explored low-GI foods based on modified starches, such as rice, breads, noodles, cheeses, and biscuits. Microcapsule technology has been widely employed to preserve volatile, light- and temperature-sensitive, and oxidizable food ingredients. This method preserves heat-sensitive SDS and RS; moreover, it was found that the use of encapsulated waxy starch instead of amylopectin-treated waxy corn starch could lead to the development of new products with high SDS levels and functional properties [85].
By replacing the shortening with polydextrose as a fat substitute, increasing the dietary fiber content using RS, and adding biscuit improvers, dairy–multigrain composite biscuits were produced [86]. The results revealed that, compared with the control, the total dietary content of the biscuits was 142.7% higher, the fat content was reduced by 35%, and the calorie content was decreased by 28.25%. Researchers used low-gluten wheat flour as the main raw material and added RS, vegetable oil, and other raw material to develop a medium GI product [87]. The hydrolysis index and GI of the RS biscuits were found to be 49.19 and 66.72, respectively.
Novelose 240 (N240, natural RS) or novelose 330 (N330, degraded RS), rather than fat, was chosen by Montesinos–Herrero et al. [88] to prepare cheese. It was established that more than half of the fat content of the prepared imitation cheese was replaced by RS, whereas the meltability remained unchanged. Scanning electron microscope (SEM) techniques were used to characterize the microstructure of the simulated cheese (Fig. 5). However, it was found that this composition caused the cheese to become harder, and RS was used to further replace up to 90% of the fat to produce 2% fatty cheese. The hardness and cohesion values are suitable for slicing, chopping, and shaping, when the water content is maintained at 60%. Although cheese has little or no fat, it has good meltability [89].
《Fig. 5》
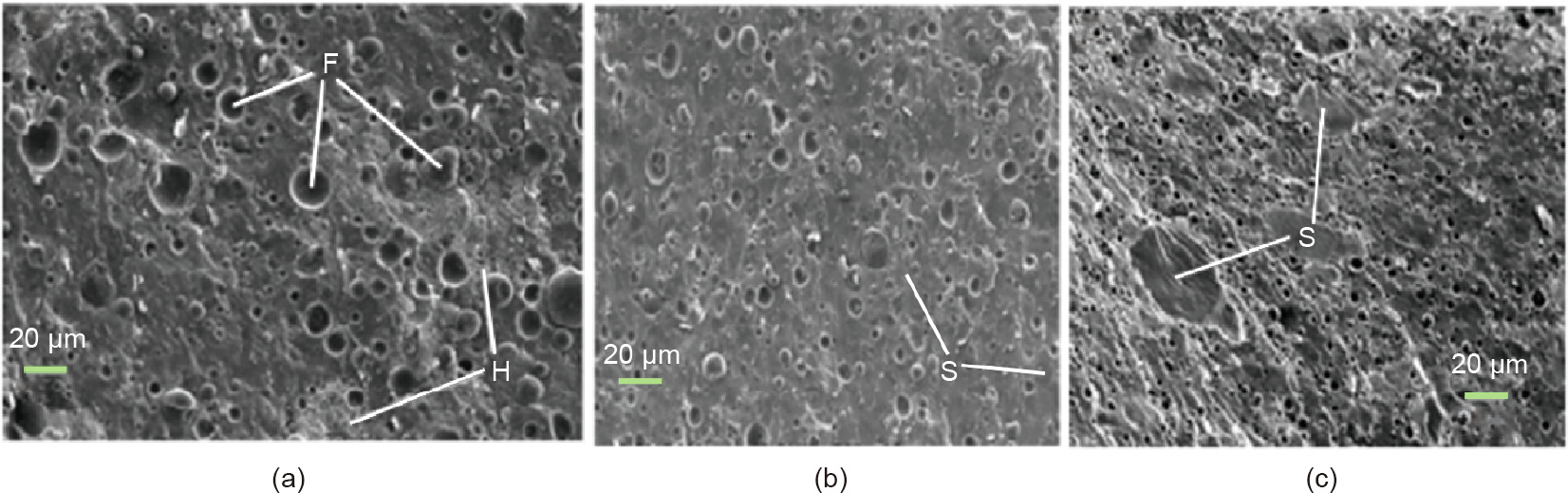
Fig. 5. SEM images (500×) of cheese with (a) 52% moisture imitation, (b) 12.5% novelose 240 (500×), and (c) 12.5% novelose 330 (800×). F: fat globule; H: honeycomb structure; S: starch. Reproduced from Ref. [88] with permission of Elsevier B.V., ©2016.
The effects of improved pea starch along with wheat flour and high levels of RS (PeaP) on bread products were assessed [90]. When the addition of PeaP in flour substitutes was as high as 10%–20%, it was beneficial in terms of a significant increase in the RS level of wheat bread (from 0.70% to 5.10%). When the flour replacement rate was as high as 20%, the mechanical, tensile, and viscous parameters were maintained without affecting the processability of the dough [91]. Overall, the in vitro digestibility of RS bread revealed that the release of reducing sugars was slow; bread with an increased RS4 concentration exhibited low in vitro starch digestibility. Moreover, the corresponding GI was much lower than that of common bread, which would be potentially vital in special diets.
The digestion behavior of starch can be modified by adding other substances. For example, the effects of phenolic compounds, proteins, and polysaccharides on starch digestion have been investigated. Some researchers have reported that gallic acid, a kind of dietary polyphenol, can be complexed with starch gel to increase the content of RS and thus decrease the predicted GI [92]. Others have found that polysaccharides, such as pullulan, can increase the total content of SDS and RS with a concomitant decrease in RDS [93]. Hydrocolloids (e.g., guar gum), another type of polysaccharide, can also reduce starch hydrolysis during in vitro digestion owing to the thickening effect, which reduces the accessibility of enzymes to a starch substrate [94]. Rice protein has been reported to be helpful in decreasing starch digestibility because of the increased ordered and aggregated structures of cooked starch and starch V-type structures [95]. In addition, the addition of oyster mushroom (Pleurotus sajor-caju) powder increased the dietary fiber content, while reducing the size of the inhomogeneous spherical starch granules formed. This interacts with the integrity of the starch granules, leading to a decrease in the sensitivity of starch to digestive enzymes and the GI of the biscuit [96].
《4.2. Design of low-GI foods via alterations in the processing conditions》
4.2. Design of low-GI foods via alterations in the processing conditions
Processing conditions have an important impact on food quality and the digestibility of the product [97]. The hardness, springiness, and chewiness of grass carp subjected to ultra-high-pressure treatment were higher than those of fresh fish and those of fish subjected to freezing treatment, indicating that ultra-high pressure can significantly improve the quality and digestibility of grass carp [98]. The dietary fiber and protein content of oats under normal steaming, autoclaving, dry roasting, and microwaving conditions are also subject to change [99].
Rice, the main source of energy for humans worldwide, is an indispensable carbohydrate in dietary nutrition. Under conditions in which rice type and milling precision are determined, the processing method is an important factor affecting the blood sugar reaction of rice food, which may further affect the digestion rate of carbohydrates by impacting the degree of ripening of rice, starch composition, and physicochemical properties, ultimately determining the glycemic-response characteristics of rice food.
Some researchers have found that soaking before cooking, adding water, and prolonging the cooking time can lead to higher rates of starch digestion during the cooking process; moreover, the starch digestion rate decreases significantly after refrigeration [100]. Further, others have explored the changes in the structure of rice under different high-temperature (80, 100, 120, and 140 °C) and pressure (0, 0.1, 0.3, and 0.5 MPa) treatments (Figs. 6 and 7) [101]. As the temperature increases, the pores in the outer layer of cooked rice decrease; moreover, the appearance of the rice (color and exterior integrity) and the texture significantly change. In contrast, the cooking pressure has a limited effect on rice. The microstructure of the outer surface and inner layer of cooked rice is shown in Fig. 6.
《Fig. 6》
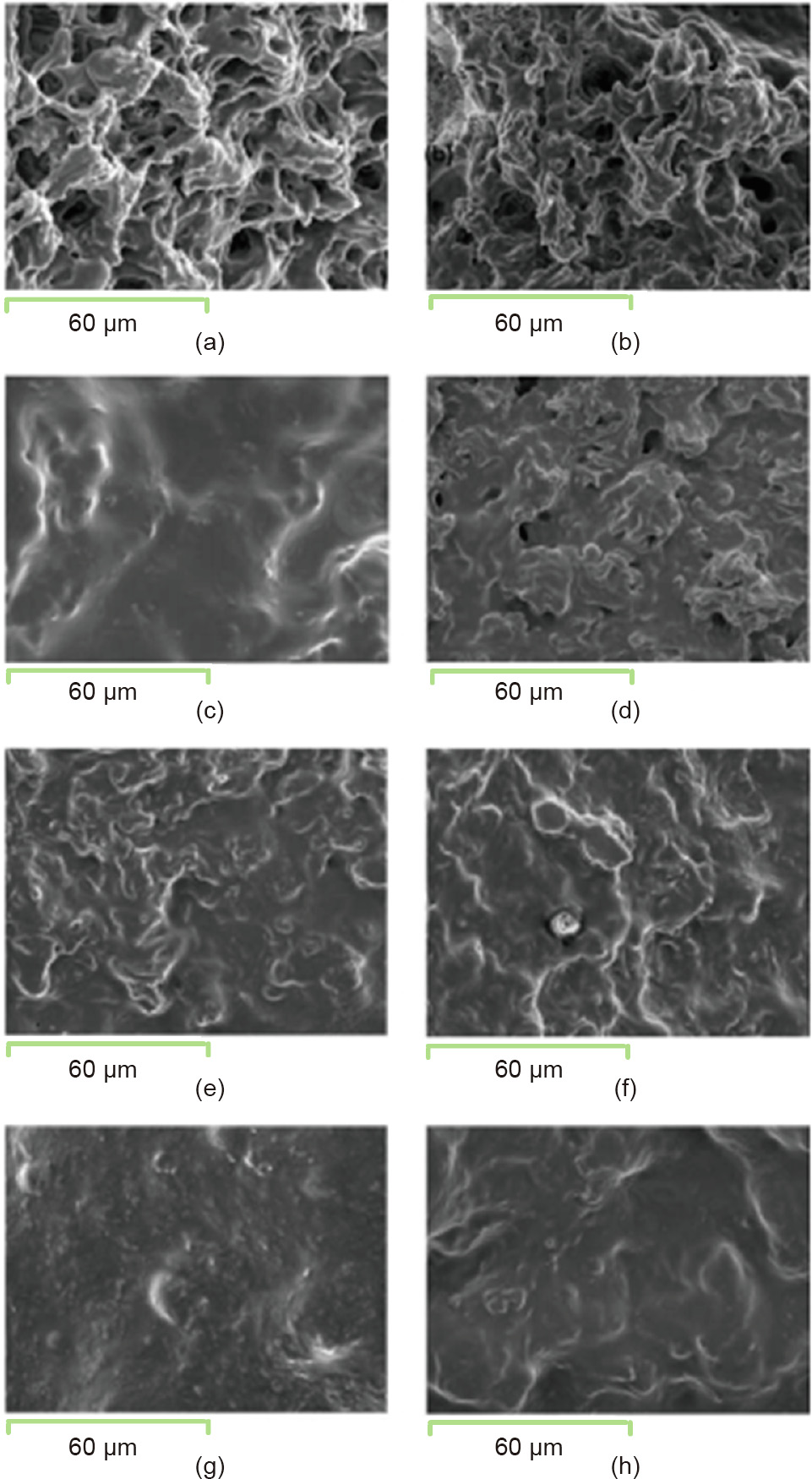
Fig. 6. SEM images of the outer surface of cooked rice under different temperatures and pressures: (a) 80 °C, 0 MPa; (b) 80 °C, 0.5 MPa; (c) 100 °C, 0 MPa; (d) 100 °C, 0.5 MPa; (e) 120 °C, 0.1 MPa; (f) 120 °C, 0.5 MPa; (g) 140 °C, 0.3 MPa; and (h) 140 °C, 0.5 MPa. Reproduced from Ref. [101] with permission of Elsevier B.V., ©2006.
《Fig. 7》

Fig. 7. SEM images of the inner layer of cooked rice under different temperatures and pressures: (a) 80 °C, 0 MPa; (b) 80 °C, 0.5 MPa; (c) 100 °C, 0 MPa; (d) 100 °C, 0.5 MPa; (e) 120 °C, 0.1 MPa; (f) 120 °C, 0.5 MPa; (g) 140 °C, 0.3 MPa; and (h) 140 °C, 0.5 MPa. Reproduced from Ref. [101] with permission of Elsevier B.V., ©2006.
The processing of convenient rice mainly includes the cooking and drying of rice, which involves the gelatinization and regrowth of starch. It was found that the RDS content of convenient rice prepared by conventional cooking was reduced to a greater extent than that prepared using a rice cooker, and the SDS content was significantly increased [102]. At the same time, it was found that rice with a low amylose content had a lower RDS content along with a higher SDS and RS content, whereas high amylose content rice had a lower SDS content and a higher RS content. Under normal temperature and pressure-cooking conditions, the cooking time of rice was shortened by approximately 30% [103]. Kinetic experiments revealed that pre-soaked rice and unsoaked rice corresponded with first-order kinetic reaction equations at room temperature and under atmospheric pressure.
Drying is also an important factor affecting the quality characteristics of rice, which mainly include moisture content, drying temperature, and surface velocity. The response surface method was used to evaluate the influence of drying conditions on the quality of rice, thereby leading to the proposition of the optimization of drying parameters [104]. These results suggest that the moisture content is the most important factor, whereas surface velocity is the least. Dry conditions have a significant influence on the texture and taste of rice. In addition, the drying temperature affects the color of rice, but it has no significant effect on the shrinkage and rehydration ability of dry rice. The morphology of dried rice at different temperatures is similar according to SEM, but it is different from that of freshly cooked rice [105].
《4.3. Prospects》
4.3. Prospects
At present, in the dietary structure of modern people, processed foods are present in abundance, whereas the intake of fruits, vegetables, and coarse grains is limited. Hence, dietary fiber is often insufficient. At the same time, the number of patients with cardiovascular disease, intestinal diseases, and diabetes increases yearly, emphasizing the importance of a diet containing less sugar, less salt, and less fat. To meet the needs of the dietary habits of consumers, Coca-Cola launched ‘‘Coca-Cola plus” in Japan in 2017 [106]. The product not only retains the original taste while featuring 0 calories but also contains 5 g of water-soluble dietary fiber, which can help reduce the absorption of fat after meals. In addition, ‘‘Sprite Fiber+” was launched in China, and its 0-calorie formulation can also meet 30% of the daily dietary fiber requirement for adults [107]. Both novel beverages are supplemented with resistant dextrin, which is a soluble dietary fiber, and it not only increases the volume of the gastrointestinal tract, causing satiety, but also accelerates bowel movements. Lowenergy and low-calorie foods are increasingly a new trend; they also provide new ideas and directions for the development of starch-based dietary foods.
《5. Conclusions》
5. Conclusions
With the urgent demand for healthy food and the increasing interest in the development of novel dietary foods, the investigation of starch has attracted considerable attention. This review summarizes the main frontier research topics related to starch from its basic chemical structure to the in vivo and in vitro determination methods of the digestibility of starch-based dietary foods. Starch is not as simple as a pure chemical agent; it is a complicated biomaterial that contains three different materials: RDS, SDS, and RS. Great strides in starch research have been made through the application of various characterization techniques, such as electron microscopy, thus allowing the direct elucidation of the micro-level morphology of starch-based dietary foods. Benefiting from the fundamental understanding of starch, novel starch-based dietary foods have been designed and synthesized to meet the requirements demanded by modern people. For instance, low-GI foods can be successfully obtained via either the direct addition of modified starch or by altering processing conditions. Therefore, in the near future, the combination of fundamental research and new processing techniques will be necessary to design and produce healthier starch-based dietary foods.
《Acknowledgements》
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31701644 and 32072268), the Science & Technology Pillar Program of Jiangsu Province (BE2018304), and the National First-Class Discipline Program of Food Science and Technology (JUFSTR20180203).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Yuxiang Bai, Xiaoxiao Li, Hangyan Ji, Yu Wang, Danni Zheng, Yanli Wang, and Zhengyu Jin declare that they have no conflict of interest or financial conflicts to disclose.













 京公网安备 11010502051620号
京公网安备 11010502051620号




