《1. Introduction》
1. Introduction
The disposal of waste plastics and scrap tyres is an extremely challenging problem in Hong Kong and worldwide because of their volume and nonbiodegradable structures [1–5]. Most of these wastes have been disposed at landfills and stockpiles [6,7] which is considered a temporary solution with various associated problems, such as potential land and water pollutions and occupation of a large land space leading to land scarcity [8,9].
Waste plastics can be generally categorized into commodity plastics [10], engineering plastics [11], and specialty plastics [12]. Among them, the volume of commodity plastics, derived from postconsumer products, such as disposable bottles, handbags, coats, umbrellas, and tableware, is largest [13,14]. The raw materials for the production of commodity plastics include mainly polyethylene terephthalate (PET), polyethylene, polypropylene, polyvinyl chloride, and polystyrene. Among them, PET plastics are the most widely used ester-based thermoplastics in beverage bottles. In 2015, the primary production of PET plastics in the world reached 68 million tonnes [15]. According to the Plastics Division of the American Chemistry Council and the Association of Plastic Recyclers, 2.782×106 t (1726 million pounds) of postconsumer PET bottles existed in 2017 in the United States; only 29.2% of them were recycled [16].
Scrap tyres from passenger cars and trucks are also major solid wastes [17]; however, they have been commonly ground into crumb rubber (CR) to produce rubberized asphalt pavements with increased performances for noise reduction, aging resistance, hightemperature stability, and fatigue resistance [18,19]. Paje et al. [20] reported that by incorporating a high content of CR into gapgraded asphalt mixtures, the tyre/road noise of an asphalt pavement could be reduced by ~2.5 decibel (dB). Wang et al. [21] demonstrated that CR could improve the low-temperature creep stiffness and high-temperature performance of virgin bitumen. The suggested dosage limit for CR was 15%–20% with respect to the weight of the virgin bitumen.
To address the waste PET problem, various studies have been carried out to recycle and reuse it in asphalt pavement construction. Most previous studies have focused on the physical recycling of PET wastes as polymer modifiers or partial replacement of fine aggregates to improve the fatigue resistance, stiffness, rutting resistance, and strength of asphalt mixes [22–24]. Modarres and Hamedi [23] applied shredded PET bottles (size: 0.425–1.18 mm) to an asphalt mixture through the dry method and reported that the PET particles improved the fatigue behavior and stiffness of the asphalt mixture. Hassani et al. [24] replaced a 3.36–4.75 mm aggregate in an asphalt mixture with waste PET granules (~3 mm) and reported that the PET-modified mixture provided a similar Marshall stability and lower Marshall flow compared to those of the control mixture without PET. However, the physical recycling of PET wastes in asphalt pavements is hindered by the limitations of potential pollutant emission at high construction temperatures and low storage stability of the PET-modified bitumen. Therefore, chemical recycling of waste PET in asphalt pavement may be considered to avoid the aforementioned issues. For waste PET, the most common chemical recycling methods include aminolysis, alcoholysis, and hydrolysis [25–27]. However, the latter two methods require higher thermal energies for the chemical reactions in addition to suitable catalysts [28,29]. Therefore, an aminolysis method has been designed to produce functionalized additives using waste PET [30]. These PET-derived additives have been applied as bitumen modifiers that can help enhance the bonding between aggregates and bitumen significantly; this mitigates the moisture damage and improves the cracking resistance of the bituminous mixture [30–32]. However, the recycling mechanism of the waste PET-derived additives and their interaction mechanisms with rubberized bitumen as a pavement material have not been sufficiently understood. To fill this information gap and develop a method to collectively recycle waste PET and waste tyre rubber into performance-enhancing modifiers for asphalt pavements, this study aims to investigate the chemical reaction mechanism for the synthesis of PET-derived additives using two amines, triethylenetetramine (TETA) and ethanolamine (EA); it also aims to characterize the performances of the PETderived additives in modifying rubberized bitumen. The PET additives synthesized with TETA and EA are denoted as PET–TETA and PET–EA, respectively.
《2. Research program》
2. Research program
To achieve the research objectives, Fourier-transform infrared (FTIR) spectroscopy and thermogravimetric analysis (TGA)– differential scanning calorimetry (DSC) were used to analyze the molecular structures, chemical compositions, and thermal properties of the additives. Subsequently, FTIR spectroscopy, viscosity, dynamic shear rheology (DSR), and multiple stress creep recovery (MSCR) tests were carried out to evaluate the modifying effects of the PET-derived additives on the rubberized bitumen. Fig. 1 shows the flowchart of the research program of this study.
《Fig. 1》

Fig. 1. Flowchart of the experimental procedures. CRMA: CR-modified asphalt binder.
《3. Materials and research methodology》
3. Materials and research methodology
《3.1. Raw materials》
3.1. Raw materials
Waste PET-based drinking bottles were collected locally and ground into small pieces of maximum size 10 mm after removing the labels and caps. Two amines, TETA and EA, supplied by SigmaAldrich Corporation, were used as chemicals to degrade the ground PET particles. The physicochemical properties of the two amines are listed in Table 1. CR from waste tyres with a size of less than 30 mesh was provided by a local supplier to prepare the rubberized bitumen. The virgin bitumen used in this study is a bitumen with a penetration grade of 60/70 (Pen. 60/70), which is commonly used in Hong Kong.
《Table 1》
Table 1 Physicochemical properties of purchased TETA and EA.

《3.2. Synthesis of PET-derived additives》
3.2. Synthesis of PET-derived additives
The PET wastes were chemically-treated with TETA and EA using the aminolysis procedure similar to that in the previous studies [30,31] to prepare the additives. The corresponding PETderived additives are denoted as PET–TETA and PET–EA, respectively. The mass ratio of PET and amine was set to 1:2. Based on preliminary trial tests, the reaction temperature and time were 140 °C and 2 h, respectively. The final PET-derived additives were obtained after vacuum filtration, repeated washing, drying at the temperature of ~20 °C, and grinding.
《3.3. Preparation of rubberized bitumen modified by PET-derived additives》
3.3. Preparation of rubberized bitumen modified by PET-derived additives
The CR particles were preheated at 120 °C for 30 min. Subsequently, 18% (with respect to the weight of virgin bitumen) CR particles were added to and mixed with the virgin bitumen at 180 °C at 3500 r·min-1 for 1 h to prepare the 18%-CR-modified asphalt binder (18CRMA). In the end, 2 wt% PET–TETA and 2 wt% PET–EA were added to 18CRMA and blended at 180 °C for 30 min to prepare 18CRMA2PET–TETA and 18CRMA2PET–EA, respectively.
《3.4. FTIR spectroscopy analysis》
3.4. FTIR spectroscopy analysis
A NicoletTM iS50 FTIR spectrometer (Thermo Fisher Scientific Inc., USA) equipped with an attenuated total reflectance detection system was used to record the characteristics of the molecular structures of PET before and after the aminolysis. The same equipment was used to characterize the physicochemical interactions between the PET additives and rubberized bitumen. As all testing materials are organic, their molecular vibrations normally occur in the middle-infrared wavenumber range of 4000–400 cm-1 . Studies indicated that 32 cycles of scanning can minimize the noise effect on the FTIR spectroscopy data collection and provide optimal spectra of organic materials [33,34]. Therefore, the following parameters were used: 32 cycles of scanning, resolution of 4 cm-1 , and wavenumber range of 4000–400 cm-1 .
《3.5. TGA–DSC》
3.5. TGA–DSC
The combination of TGA and DSC is a common methodology to analyze the chemical compositions and thermal properties of materials [35]. TGA and the corresponding derivative thermogravimetry (DTG, dw/dT) were used in this study to determine the chemical compositions, decomposition temperatures and rates, and thermal stabilities of the PET wastes before and after the chemical treatment. The results obtained by the DSC tests on the waste PET and its additives were analyzed to obtain their thermal parameters, including the crystal transition point, melting temperature, and pyrolysis temperature. The test temperatures were controlled between 50 and 600 °C with an increase rate of 20 °C·min-1 in nitrogen atmosphere.
《3.6. Rotational viscosity test》
3.6. Rotational viscosity test
The workability of each binder was characterized by an rotational viscosity (RV) test at 135 and 180 °C using an NDJ-1C Brookfield rotational viscometer (AMETEK Inc., USA) in accordance with American Society for Testing and Materials (ASTM) D4402 [36].
《3.7. DSR test》
3.7. DSR test
According to the data provided by the Hong Kong Observatory in 2018, the mean temperatures of Hong Kong vary in the range of 6.8–35.4 °C [37]. Thus, the deformation resistances of the studied binders were evaluated in the medium temperature range of 8–35 °C. DSR tests were carried out at a fixed oscillatory frequency of 10 rad·s-1 in the range of 35–8 °C with a pair of 8 mm plates using an MCR 702 DSR apparatus produced by Anton Paar Inc. (USA) [38]. According to ASTM D7175 [39] to record the complex modulus (G* ) and phase angle (δ), the binder samples were subjected under the aforementioned conditions with a controlled strain of 1%, which were set to ensure that the tests are carried out in a linear viscoelastic region. For the fatigue factor (G* sinδ) analysis, the binder samples underwent a pressurized aging vessel process in accordance with ASTM D6521 [40] before testing under the aforementioned conditions stipulated by ASTM D7175 [40].
《3.8. MSCR characterization》
3.8. MSCR characterization
To better understand the permanent deformation resistances and elastic recovery performances of the studied binders under various applied loads at high temperatures, MSCR tests using the DSR apparatus were carried out in accordance with American Association of State Highway and Transportation Officials (AASHTO) T350 [41,42]. All binders were initially short-term-aged by the rolling thin-film oven test in accordance with ASTM D2872 [43]. These aged binders were creep-tested at two stress levels of 0.1 and 3.2 kPa for ten cycles (creep: 1 s; recovery: 9 s). Based on the MSCR test results, the percent recovery (R) and nonrecoverable compliance (Jnr) of each binder were calculated by
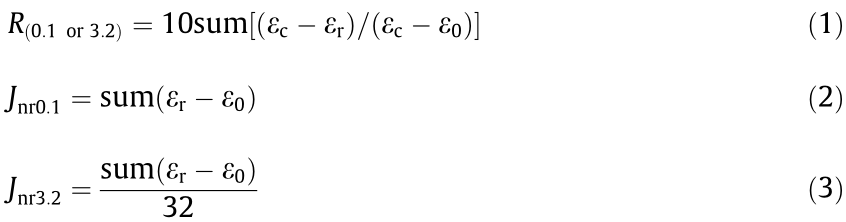
where ε0 is the strain at the start of the creep portion of each cycle, εc is the strain at the end of the 1 s creep portion of each cycle, and εr is the strain at the end of the 9 s recovery portion of each cycle.
《4. Results and discussion》
4. Results and discussion
《4.1. FTIR spectroscopy analysis of the PET-derived additives》
4.1. FTIR spectroscopy analysis of the PET-derived additives
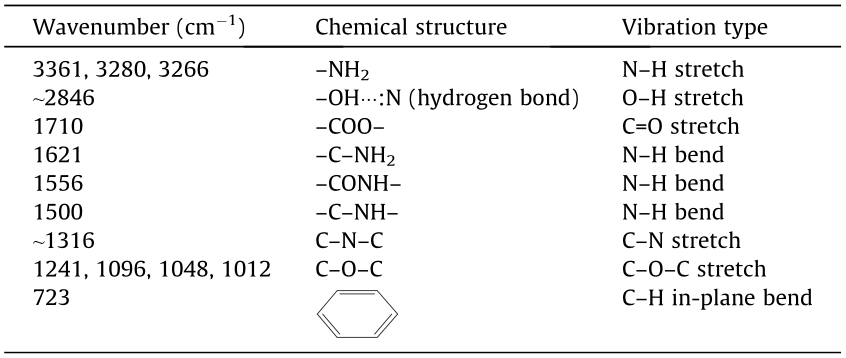
Fig. 2 presents the FTIR spectroscopy test results for the waste PET and two PET-derived additives. The corresponding attributions and vibration types of the major characteristic bands of each material are presented in Table 2. The molecular structure of PET was degraded after the aminolysis treatment. After the chemical treatment by TETA or EA, the major carbonyl group (C=O) band of PET at 1710 cm-1 was not observed, whereas the ether group (C–O–C) band at 1245–1010 cm-1 was significantly diminished. In addition, the intensity of the spectrum band at 723 cm-1 , attributed to the in-plane bending vibration of C–H sourced from the benzene of PET, was significantly smaller after a series of degradation reactions caused by the chemical treatment with TETA or EA. These results indicate that the waste PET can be thoroughly degraded to small molecules by chemicals with active amino and hydroxyl groups.
In Fig. 2, the spectrum bands of PET–TETA at 3266 and 1621 cm-1 are attributed to the stretching and bending vibrations of N–H of –NH2, respectively. The spectrum bands at 1556 and 1500 cm-1 are attributed to the bending vibrations of N–H on –CONH– and –C–NH–, respectively. The band at ~1316 cm-1 represents the stretching vibration of C–N of C–N–C. The band around 2846 cm-1 is attributed to the stretching vibration of –OH of hydrogen bonds (–OH···:N). The major characteristic bands of PET–EA, different from those of PET–TETA, are located mainly at 3361, 3280, and ~1048 cm-1 , which are attributed to the antisymmetric and symmetric stretching vibrations of N–H of –NH2 and stretching vibration of C–O–C, respectively.
《Fig. 2》

Fig. 2. FTIR spectra of waste PET and PET derived additives.
《Table 2》
Table 2 Major characteristic bands in FTIR spectra of waste PET and its additives.
The changes in characteristic structures of PET caused by the chemical treatment by TETA and EA can be explained by the balland-stick models, as illustrated in Fig. 3. For the TETA treatment, the primary and secondary amine groups (–NH2 and –NH–) in TETA can actively attack the ether groups (–COO–) on the main chain of PET to produce substances with the characteristic structures of –CONH– and –CON–. For the EA treatment, owing to the terminal groups of –NH2 and –OH in EA, the reaction products are attached to the newly formed structures of –CONH– and C–O–C.
《Fig. 3》
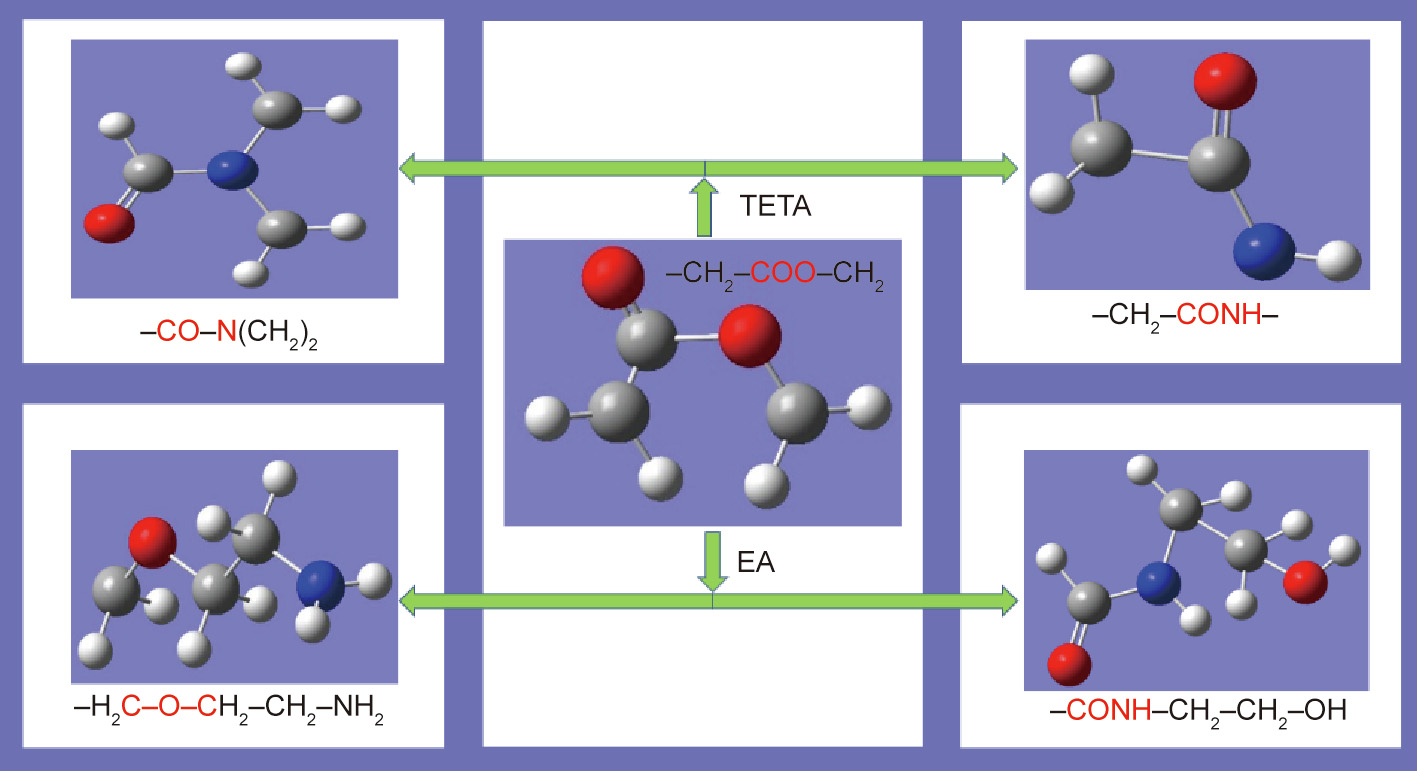
Fig. 3. Ball-and-stick models displaying main changes in characteristic structures of PET treated by TETA and EA.
《4.2. Thermal analysis of the PET-derived additives》
4.2. Thermal analysis of the PET-derived additives
TGA, DTG, and DSC are commonly used to evaluate the thermal properties of materials [35,44]. In this study, these methods were used to understand the thermal behaviors of the PET-derived additives and determine their physicochemical conditions during the hot mixing of the bituminous mixtures. Figs. 4 and 5 show the thermal behaviors of the waste PET, PET–TETA, and PET–EA based on the test results of the TGA/DTG and DSC, respectively. Fig. 4(a) shows that, after the chemical treatment, the initial decomposition is shifted to lower temperatures and that various stages of mass loss exist, which correspond to the peaks in the DTG curves in Fig. 4(b). With increase in temperature, TETA is more effective in breaking the molecular structure of PET and has more significant effects on the mass-loss ratios at certain temperatures compared to EA. Both additives exhibit three stages of mass loss. These results imply that both PET-derived additives contain three components with different thermal properties, instead of one component. The thermal stability of PET–TETA is not as good as that of PET–EA, because TETA can provide more sites for reaction with the ester groups of PET and cause a more significant PET degradation owing to the large numbers of –NH– and –NH2 active groups compared to EA, as demonstrated by the aforementioned FTIR spectroscopy analysis.
《Fig. 4》

Fig. 4. Thermal analyses of waste PET and PET derived additives: (a) TGA and (b) DTG. T: temperature; dm/dt: mass loss rate.
Fig. 5 shows the endothermic and exothermic effects of the waste PET and its derived additives. For the waste PET, the small endo-peak at 245 °C and exo-peaks at 424 and 472 °C correspond to the melting and pyrolysis temperatures, respectively. For PET– EA, the endo-peaks at 199 and 235 °C correspond to the crystal transition point and melting temperature, respectively, which implies that this additive behaves as crystal particles at a temperature lower than 199 °C and starts to melt around 235 °C. An exo-peak of PET–EA is observed around 341 °C, which corresponds to the pyrolysis temperature. This indicates that the additive strongly decomposes at this temperature. For PET–TETA, the first peak around 122 °C does not correspond to the crystal transition temperature or melting temperature. It corresponds to the decomposition temperature of one of the components in the additives. These results confirm that some of the unstable small molecules in PET–TETA can be decomposed during the mixing with the rubberized bitumen at temperatures of 175–180 °C [45,46] whereas PET–EA simply acts as a functionalized filler in the binder because of its high melting temperature of ~235 °C. The different characteristics of PET–TETA and PET–EA in the DSC tests are observed mainly as TETA has more amine groups than EA in its molecular structure. This enables a better reaction with the ester groups in PET and leads to a more serious degradation of PET.
《Fig. 5》
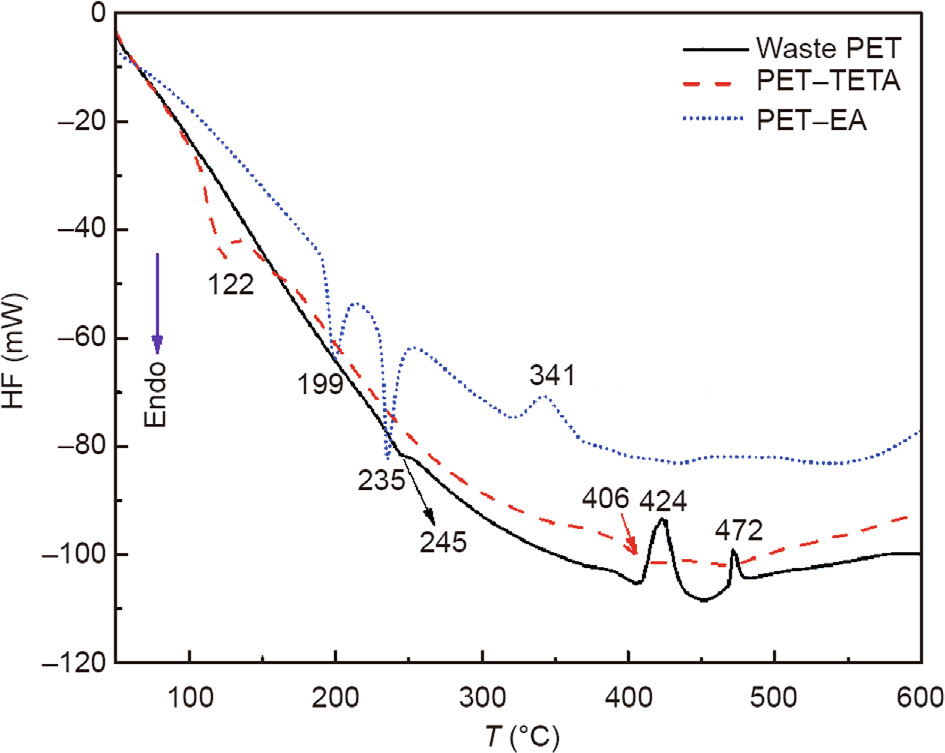
Fig. 5. DSC plots of waste PET and PET derived additives. HF: heat flow; T: temperature.
Table 3 summarizes the thermal parameters obtained by the TGA, DTG, and DSC tests. PET–TETA contains three chemical components with relatively low maximum decomposition temperature (Td,max) values of 122, 252, and 406 °C. The initial decomposition temperature (Ti) and final decomposition temperature (Tf) values of PET–TETA are 99.2 and 499.5 °C, respectively, which indicates that it can be easily decomposed with temperature increase. For PET–EA, all thermal parameters are higher than those of PET–TETA, particularly Ti (275.4 °C). Overall, PET–TETA is less thermally stable than PET–EA.
《Table 3》
Table 3 Thermal parameters from TGA, DTG, and DSC.

dm/dt: mass loss rate; dm/dt@max: maximum mass loss rate at stages.
《4.3. Synthesis reaction mechanism analysis of the PET-derived additives》
4.3. Synthesis reaction mechanism analysis of the PET-derived additives
Through the physicochemical analyses by the FTIR spectroscopy and TGA–DSC tests, valuable information on the PET-derived additives, including the disappearance of existing groups and appearance of new groups, chemical components, and thermal decompositions, can be obtained and synthesized to help better understand their synthesis reaction mechanisms.
Figs. 6 and 7 illustrate the reaction mechanisms for the productions of PET–TETA and PET–EA, respectively. PET has a long molecular chain with the main characteristic structures of ester groups, which can be destroyed through various chemical treatments, such as aminolysis [3], alcoholysis [47], and hydrolysis [48]. During the reaction between PET and TETA, the ester groups on the main chain structure of PET suffer from the focused attacks of –NH– and –NH2 in TETA, which breaks the macromolecular structures. Small molecules (Figs. 6(i)–(iii)) with new linkages of –CONH– and –CO–N(CH2)2 and ethylene glycol (Fig. 6(iv)) are then formed. After removing the ethylene glycol using water, the PET–TETA additive is obtained. Its characteristic structures and chemical components are consistent with the FTIR spectroscopy and TGA–DSC results.
《Fig. 6》
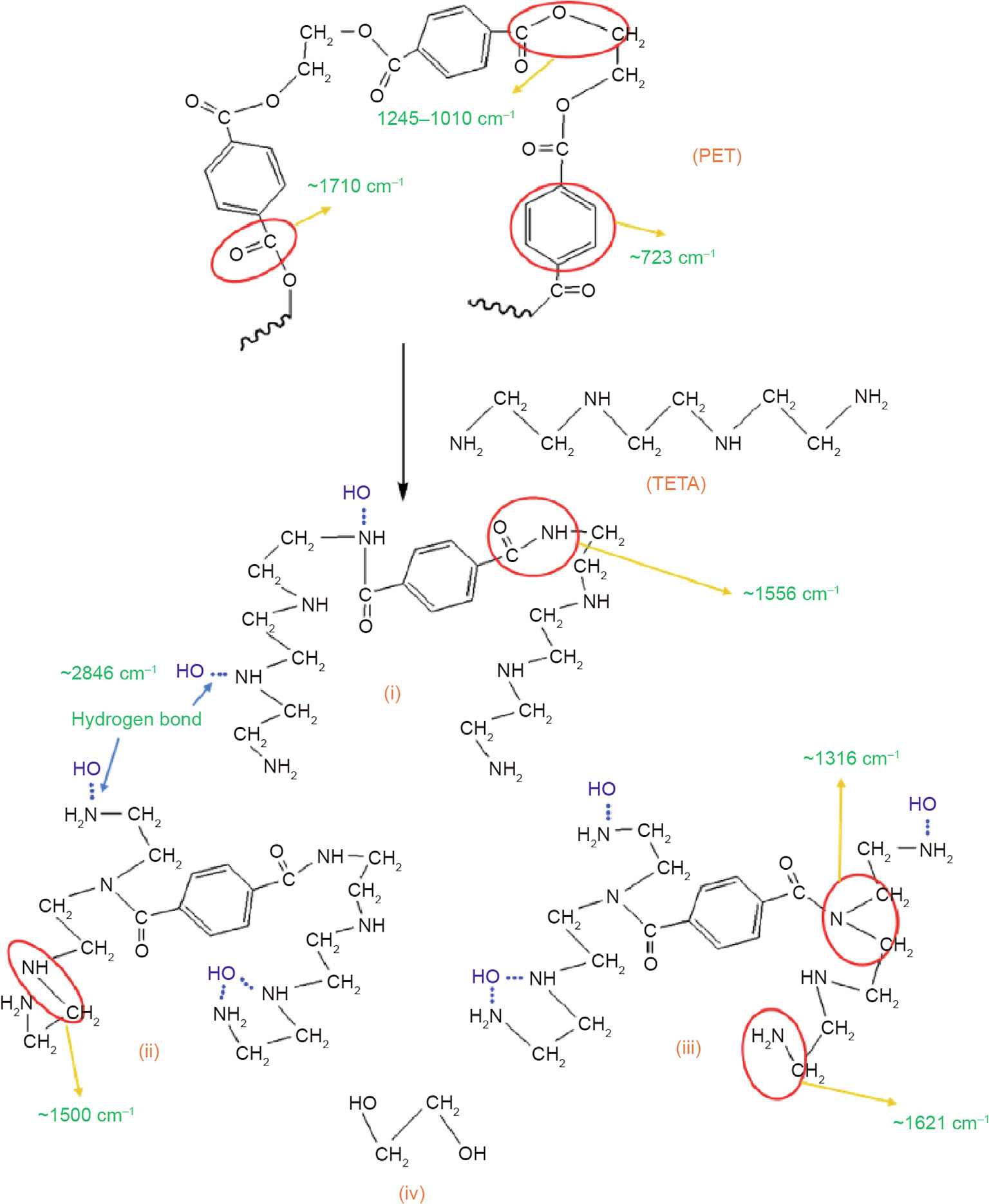
Fig. 6. Reaction mechanism of synthesizing PET–TETA additives. (i), (ii), and (iii) represent PET additive; and (iv) is ethylene glycol.
Similar to TETA, EA has various molecular structures with terminated groups of primary amine (–NH2) and hydroxyls (–OH), which have high probabilities to meet and react with the ester groups in PET at high temperatures. The terminal –NH2 groups of EA can react with the –COO– groups and finally form a new linkage of –CONH–, while its terminal groups of –OH also react with –COO– groups through ester exchange. Owing to these reactions, new products are obtained, as shown in Figs. 7(i)–(iii). However, around 1700 cm-1 , no new peak is observed in the FTIR spectrum of PET–EA. Thus, it is very likely that the new products in Figs. 7(ii) and (iii) are interim products. As the reaction continues, these interim products transition to new more-stable products (Figs. 7(v) and (vi)) with removed C=O, which can be verified by the FTIR spectroscopy test results.
《Fig. 7》
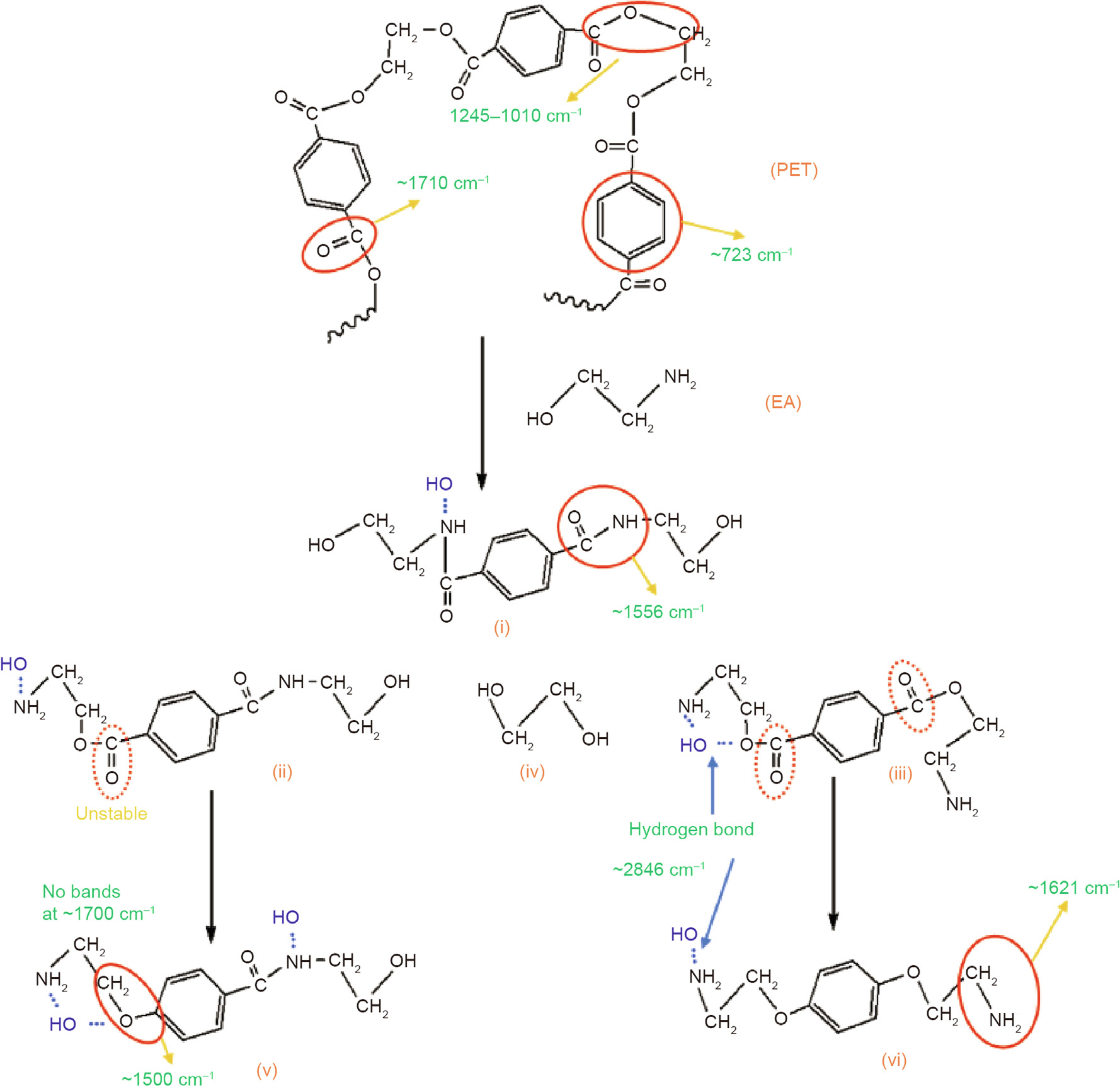
Fig. 7. Reaction mechanism of synthesizing PET–EA additives. (i), (v), and (vi) represent PET additive, (ii) and (iii) are interim products, and (iv) is glycol.
《4.4. Workabilities of the rubberized binders with the PET-derived additives》
4.4. Workabilities of the rubberized binders with the PET-derived additives
Viscosity tests were carried out to determine the workabilities of the rubberized binders containing the PET-derived additives. The viscosity test results for these binders at 135 and 180 °C are shown in Fig. 8. The incorporation of 2% PET–TETA or PET–EA does not help reduce the viscosity of 18CRMA at both temperatures. In other words, regardless of the additive type, the dosage of 2% cannot improve the workability of 18CRMA and the viscosity–temperature relationships of the modified binders remain close to that of 18CRMA. This occurs because the high amount of CR expands to relatively large volumes by absorbing the light fractions of the virgin bitumen. A small amount of PET additives cannot effectively change the viscosity of the binder except for the chemical reactions and absorption. However, overall, the PET additives are considered to have no negative effects on the hot-mix processing of asphalt materials.
《Fig. 8》
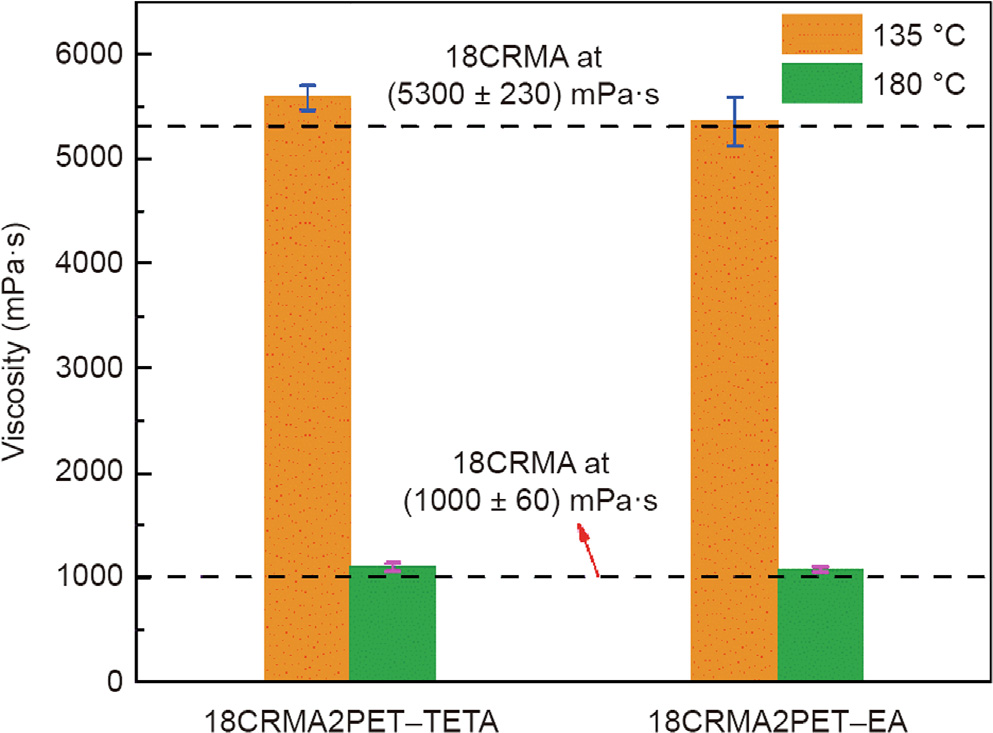
Fig. 8. Viscosity changes of CRMA with PET derived additives at various temperatures.
《4.5. FTIR spectroscopy analysis of the rubberized binders modified by the PET-derived additives》
4.5. FTIR spectroscopy analysis of the rubberized binders modified by the PET-derived additives
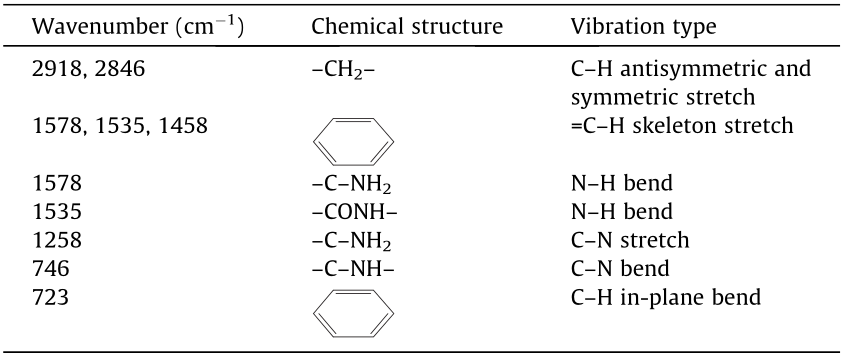
To determine whether the PET-derived additives can react with the chemical fractions in the rubberized binders, FTIR spectroscopy tests were carried out on 18CRMA, 18CRMA2PET–TETA, and 18CRMA2PET–EA. The FTIR spectra of these binders are shown in Fig. 9. Their major characteristic changes in bands are presented in Table 4. The main changes after adding PET–TETA and PET–EA into CRMA occur at 1578 and 1535 cm-1 , which are attributed to the skeleton vibration of benzene, and at 723 cm-1 , caused by the C–H in-plane bending vibration of benzene. The intensities of the peaks at these locations are notably decreased, particularly for 18CRMA2PET–TETA. For CRMA, the peak at 746 cm-1 attributed to the C–N bending vibration of –C–NH– may originate from the vulcanizing coagents, whose intensity increases with the addition of the PET-derived additives. This result does not provide evidence to confirm any chemical reaction between the additives and rubberized bitumen. Instead, the physical interactions between both PET-derived additives and rubberized bitumen are significant owing to the effects of the –NH2 and –NH-based structures in the additives. The physical interactions between the PET additives and rubberized bitumen originate mainly from intermolecular forces, such as the van der Waals forces and hydrogen bonds. Some polarized molecular groups in rubberized binder, such as hydroxyls and carboxyls, can physically interact with the amine structures in the additives. The FTIR spectroscopy results do not provide evidence that the presence of the CR has a significant effect on such physical interactions.
《Fig. 9》

Fig. 9. FTIR spectra of CRMA with PET derived additives.
《Table 4》
Table 4 Major characteristic bands in FTIR spectra of the studied binders.
《4.6. Complex modulus, phase angle, and fatigue factor》
4.6. Complex modulus, phase angle, and fatigue factor
Fig. 10 shows that PET–TETA significantly decreases the complex modulus and increases the phase angle of 18CRMA in the medium temperature range, whereas PET–EA slightly improves the complex modulus of 18CRMA and decreases its phase angle. These results indicate that PET–TETA introduces more viscous characteristics to 18CRMA, whereas PET–EA makes 18CRMA slightly more elastic. This occurs because during the binder preparation at a high temperature one of the components in PET–TETA can be easily decomposed into smaller molecules and well dispersed into the binder in the liquid state because of the lower decomposing temperature, which provides viscous characteristics. All components of PET–EA are more thermally stable as solids owing to their high decomposition temperatures, which hardens the binder, as demonstrated by the aforementioned thermal analysis results.
《Fig. 10》
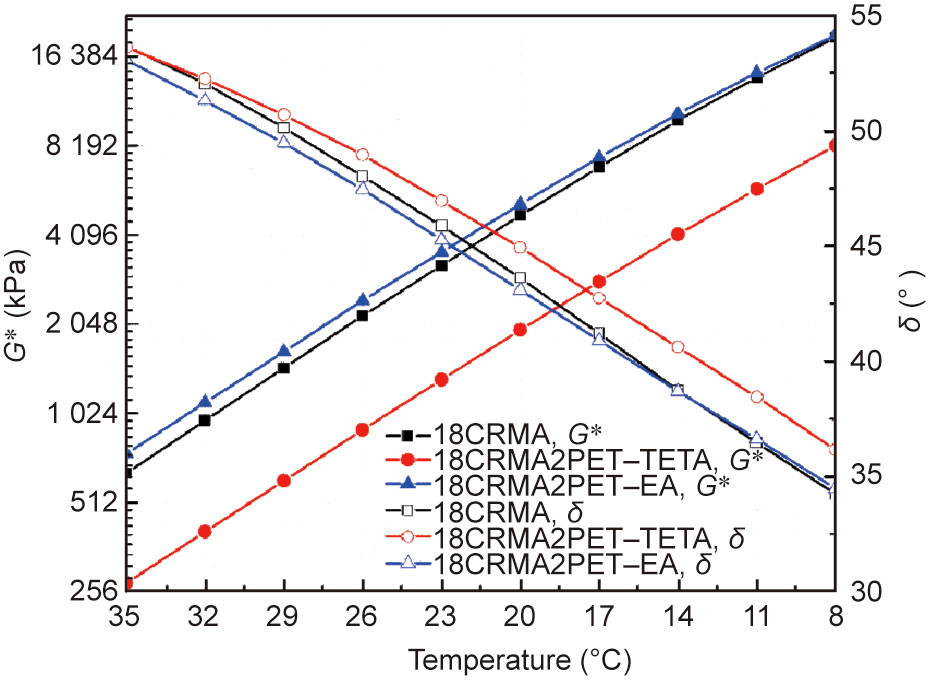
Fig. 10. Complex modulus and phase angle of 18CRMA, 18CRMA2PET–TETA, and 18CRMA2PET–EA.
Fig. 11 shows the fatigue factor test results, which can be used to compare the fatigue resistances of the studied binders after long-term aging. According to the Superpave specification in AASHTO M320 [49] the failure temperatures, which are temperatures corresponding to the G* sinδ value of 5000 kPa, were determined, as presented in Table 5. PET–TETA significantly reduces the fatigue factor of 18CRMA, which decreases the failure temperature from 16.3 to 10.5 °C, whereas PET–EA almost does not affect the fatigue factor and failure temperature. These findings indicate that PET–TETA provides a significant positive effect on the fatigue resistance of 18CRMA, which is not observed for PET–EA.
《Fig. 11》
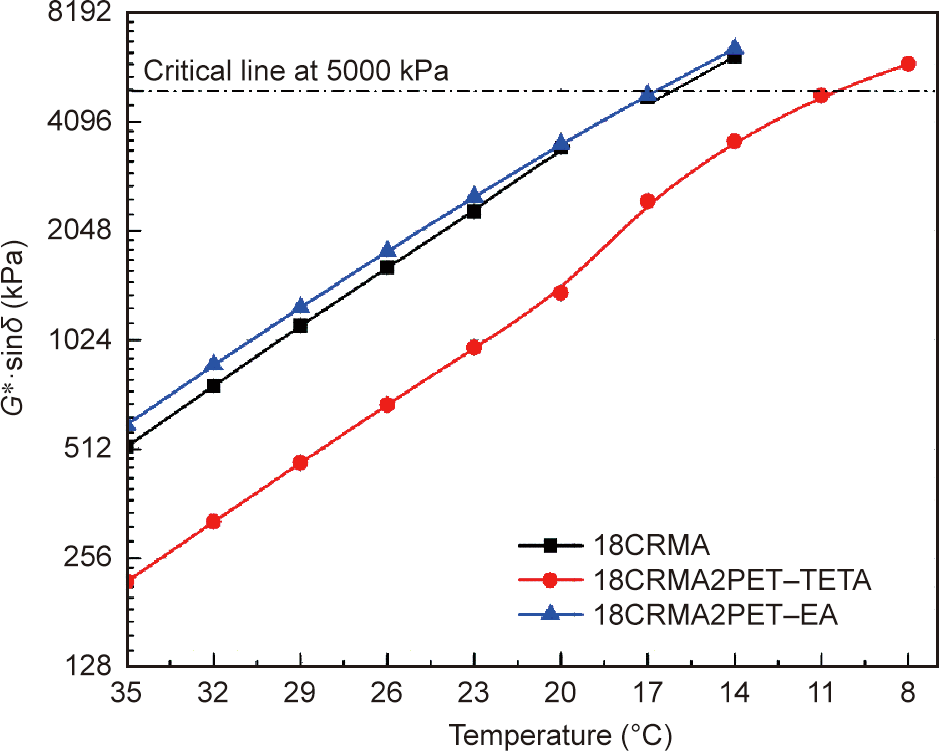
Fig. 11. Fatigue factor of 18CRMA, 18CRMA2PET–TETA, and 18CRMA2PET–EA.
《Table 5》
Table 5 Failure temperature of the studied binders when G* sinδ reached 5000 kPa.

《4.7. MSCRs of the rubberized binders modified by the PET-derived additives》
4.7. MSCRs of the rubberized binders modified by the PET-derived additives
The accumulated strains revealing the permanent deformations of the studied binders at 64 °C and controlled stresses of 0.1 and 3.2 kPa are plotted in Fig. 12. At both stress levels, PET–TETA leads to an increased accumulated strain of 18CRMA, whereas PET–EA causes the opposite effect. This indicates that PET–TETA cannot increase the permanent deformation resistance of 18CRMA, whereas PET–EA can increase it. This occurs mainly because the partial solid-phase PET–TETA changes to semiliquid substances and disperses into the binder at 64 °C, whereas PET–EA acts as a filler loaded into the binder, as verified by the previous thermal analysis results.
《Fig. 12》

Fig. 12. Accumulated strains of 18CRMA, 18CRMA2PET–TETA, and 18CRMA2PET– EA with controlled stresses of (a) 0.1 kPa and (b) 3.2 kPa at 64 °C.
The percent recoveries (R) and nonrecoverable compliances (Jnr) of the studied binders are presented in Figs. 13 and 14, respectively. At 0.1 kPa, R0.1 of 18CRMA is decreased by 11.0%, whereas Jnr0.1 is increased by 0.038 kPa-1 after PET–TETA is added. R0.1 is increased by 3.8%, whereas Jnr0.1 is decreased by 0.009 kPa-1 when PET–EA is added. These results imply that PET–TETA makes 18CRMA more stress-susceptible at a small stress level and weakens its anti-deformation property at 64 °C, whereas PET–EA has lesser effects on the stress susceptibility and elastic recovery of 18CRMA. However, when the applied stress is changed to 3.2 kPa, R3.2 of 18CRMA does not significantly change with the incorporation of both additives. When PET–TETA and PET–EA are added, Jnr3.2 of 18CRMA only slightly changes from 0.285 to 0.314 and 0.264 kPa-1 , respectively. Both values of Jnr3.2 meet the E-grade requirement (Jnr3.2 smaller than 0.5 kPa-1 ) according to AASHTO M 332 [50]. These results indicate that the addition of both additives has insignificant effects on the change in the susceptibility of 18CRMA under a high stress and does not reduce the good bearing capacity of 18CRMA under a heavy traffic.
《Fig. 13》

Fig. 13. Percent recovery of 18CRMA, 18CRMA2PET–TETA, and 18CRMA2PET–EA at 0.1 and 3.2 kPa.
《Fig. 14》

Fig. 14.Jnr of 18CRMA, 18CRMA2PET–TETA, and 18CRMA2PET–EA at 0.1 and 3.2 kPa.
《5. Findings and conclusions》
5. Findings and conclusions
In this study, two waste PET-derived additives, PET–TETA and PET–EA, were produced and their molecular structures, thermal properties, and synthesis mechanisms were characterized. Their performances in modifying rubberized bitumen containing 18% CR were evaluated through both chemical and rheological analyses. The findings of this study can be summarized as follows.
(1) The FTIR spectroscopy and TGA–DSC results revealed that both PET-derived additives contained three components with functional amine-based groups. PET–TETA was degraded to smaller molecules, whereas PET–EA remained stable as a filler during the modified-binder preparation at high temperatures.
(2) The analyses on the additive synthesis mechanisms showed that the ester groups (–COO–) of PET were destructed, which caused chain breaking; small molecules with new linkages of –CONH– and –CO–N(CH2)2 were formed owing to the attacks by the amine-based groups in TETA and EA.
(3) The FTIR spectroscopy analyses indicated that the main interactions between the PET-derived additives and rubberized bitumen were physical.
(4) The workability of the rubberized bitumen was not significantly affected when small amounts (2%) of the PET-derived additives were incorporated.
(5) PET–TETA significantly increased the fatigue resistance of the rubberized bitumen, whereas PET–EA improved its deformation resistance.
(6) According to the MSCR test results, when PET-derived additives were added, the rubberized bitumen could maintain its original deformation resistance at 64 °C and high stress of 3.2 kPa.
In general, this study shows that waste PET can be chemically upcycled into functional additives, which can increase the overall performance of rubberized bitumen. The recycling method developed in this study can not only alleviate the landfilling problems of both waste PET and scrap tyres, but also turn these wastes into value-added new materials for building more durable pavements. Further studies can be carried out on the performances of bituminous mixtures incorporated with the two waste materials in both laboratory and field.
《Acknowledgements》
Acknowledgements
The authors would like to acknowledge the funding support from the Hong Kong Environment and Conservation Fund through ECF Project (84/2017) and Science and Technology Project of Henan Provincial Department of Transportation (2020J6).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Xiong Xu, Zhen Leng, Jingting Lan, Wei Wang, Jiangmiao Yu, Yawei Bai, Anand Sreeram, and Jing Hu declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




