《1. Introduction》
1. Introduction
The prevalence and incidence of healthcare concerns in children have continued to increase in recent decades, including metabolic-, allergic-, and auto-immune-disorder-related diseases [1–5]. However, efficient treatments for these diseases remain scarce. Meanwhile, modern medical techniques such as antibiotics are a ‘‘double-edged sword,” in that they are of great help in enhancing the survival rate and decreasing the incidence of pediatric diseases, but can destroy the normal microbial ecology inside a child’s gut. The occurrence of pediatric diseases is multi-factorial, including genetics [6,7], infection [8–10], and immunity [11–14]. With research progress, an increasing amount of evidence points to the gut microbiota of early life as one of the risk factors contributing to these diseases, which are dependent on genetics, nutrition, and other environmental factors [15–17].
It is widely accepted that the human microbiota plays an important role in health and diseases [18]. Modern lifestyle factors, such as maternal diet, gestational age at birth, mode of delivery, feeding mode, and antibiotic exposure, can disturb the normal colonization and maturation of the human gut microbiota during early life [19,20]. It is notable that microbial interventions of microbiota, including the use of probiotics and prebiotics, have been shown to be potentially effective management tools that can optimize the microbiota of infants and children [21–24]. Although the safety of probiotics in infants and children requires further clarification [25,26], the role of the early life microbiota in human growth and development is becoming a hot topic [27], while remaining unclear.
Human early life microbiota is a key ‘‘window” period for manual intervention to establish a healthy microbiota, since the gut microbiota of infants changes rapidly and will reach a relatively stable state within the first three years of life [28,29]. The gastrointestinal (GI) tract contains the largest and most diverse bacterial communities in the human body (typically 1011–1012 microbes·mL-1 of intestine) [29]. It can promote the maturation of physiological development (e.g., the synthesis of vitamin K) and functions for immunity and pathogen resistance throughout an individual’s life [17]. Microbial dysbiosis has been shown to cause metabolic abnormalities and immune disorders, especially in infants and young children. It leads to metabolic-, allergic-, and auto-immune-disorder-related diseases, such as obesity [30], type 1 diabetes (T1D) [31], allergies [32], autism [33], inflammatory bowel disease (IBD) [34], and stunting [19].
In addition to its physiological functions, the microbiota’s involvement in the healthy development of a child’s immune system and the central nervous system (CNS) during neurodevelopment is attracting scholarly attention. First, the crosstalk in the gut, where the greatest number and most diverse immune cells meet trillions of microbes [35], is crucial to host immune homeostasis. The gut contains multiple immune cell subsets, and the innate immunity of the host is well orchestrated in the gut. Second, the contribution of the gut microbiota to the CNS of children through the microbiota–gut–brain axis is extremely important [36].
Studies have demonstrated that neurodevelopment in infancy is susceptible to internal and external microbial factors [37–39]. For example, exposure to pathogens (e.g., Campylobacter jejuni or Escherichia coli) can lead to abnormal behaviors, including anxiety-like behaviors and memory disorders, in young mice [37,38]. In addition, microbial metabolites—that is, short-chain fatty acids (SCFAs)—can promote the maturation of the microglia and help the microglia maintain their normal functions [40]. Moreover, nutritional components, probiotics, prebiotic oligosaccharides, and certain amino acids have a neuroprotective effect on white-matter damage of the brain by regulating inflammation and infection, which may follow preterm birth [41].
《2. Features of the gut microbiota from infancy to childhood》
2. Features of the gut microbiota from infancy to childhood
A normal gut microbiota plays an important role in human health, including nutrient acquisition, immune regulation, neurodevelopment, and behavior [18,27,42]. Infancy and childhood are the most important periods for the shaping and maturation of the intestinal microbiota [43]. The predominant microbiota of infants and children change rapidly during the first three years after birth. For example, microbiota colonize the intestine of infants immediately after birth, and the trajectory of the gut microbiota shows multiple resemblances across all infants [43–45]. The gut microbiota of infants will reach an adult-like composition at one year of age, and it may take about 2.5–3 years for infants to establish a stable adult-like intestinal microbiota community [28,46]. Before a stable microbiota is constructed in children, there are three distinct stages: the development stage (3–14 months), transition stage (15–30 months), and stability stage (≥31 months) [47]. Bifidobacterium is the predominant bacterium during the developmental stage [47,48]; two phyla of the Proteobacteria and Bacteroidetes change significantly in the transitional stage [46–48]; and Firmicutes is dominant in the stable stage [46,47,49]. Furthermore, healthy children are rich in Bifidobacterium, Faecalibacterium, and Lachnospiraceae, while adults have abundant Bacteroides [27].
From a functional perspective, there is a major change in genes related to synthetic nutrients, amino acid degradation, oxidative phosphorylation, and mucosal inflammation between non-adults and adults [27]. The intestinal microbiota of children is more likely to support functions related to ongoing development and antiinflammatory properties, including vitamin B12 synthesis and folate metabolism [48,50]. The intestinal microbiota of adults is enriched with the genes of oxidative phosphorylation, lipopolysaccharide (LPS) biosynthesis, flagella assembly, and steroid hormone biosynthesis [27,51,52]. These features of an adult microbiota have been suspected to be the reason for the increased incidence of obesity and metabolic disorders [48,52].
《3. Factors shaping the gut microbiota from infancy to childhood》
3. Factors shaping the gut microbiota from infancy to childhood
Different microbiota colonization patterns of infants and children affect the subsequent regulation of immune responses and potential disease incidence in their lifetime [53–55]. A growing body of evidence has shown that, during the establishment stage (2.5–3 years) of a child, certain factors can shape the structure and composition of the gut microbiota, including maternal prenatal condition, delivery mode, breast or bottle feeding, early diet, and postnatal medical interventions [19,56].
《3.1. Delivery mode》
3.1. Delivery mode
Delivery mode is the most important determining factor for the establishment of a healthy microbiome in early life. Newborns delivered by Cesarean section are first colonized with skin microbes from their mothers, and the microbes in all parts of these newborns are similar to the skin communities of their mothers [57]. Similarly, the bacterial communities in each part of vaginally delivered infants resemble the vaginal communities of the mothers [57]. Interestingly, although infants delivered by Cesarean section and those from vaginal delivery present a similar level of microbiota maturity in the first six months of life, the maturation of the microbiota in Cesarean-section born infants stagnated in the next six months, compared with vaginally born infants [58]. In the subsequent year, the diversity of microbiota in Cesareansection born infants gradually matured and resembled with those of vaginally born infants [58]. However, significant differences are observed in the predominant microbiota of infants with different delivery modes. For example, in the first three months of life, infants delivered by Cesarean section had a lower level of Actinobacteria and Bacteroidetes and a higher level of Firmicutes when compared with vaginally delivered infants, which were predominantly colonized by Bifidobacterium, Bacteroides, Clostridium, and Lactobacillus [55]. Research has shown that infants born by Cesarean section have a higher incidence of T1D [59], obesity [60], asthma [61], celiac disease [59], and childhood death [61] in comparison with vaginally delivered infants. Thus, delivery modes impact the gut microbiota in the first year after birth.
《3.2. Feeding practice》
3.2. Feeding practice
Feeding practice is another determining factor for the composition of the gut microbiome in early life. It is well known that breast feeding is beneficial to shape the intestinal microbiota in infants. Breast feeding can directly provide the infant with probiotics from the mother while indirectly promoting the growth of beneficial Bifidobacterium in the gut by giving the infant human milk oligosaccharides as prebiotics [62–66]. The microbial composition of infants who are breastfed is different from that of infants who are formula fed [64,67]. In their first three months of life, the maturity of their microbiota, phylogenetic diversity, and growth rate of bacterial richness in formula-fed infants are significantly reduced when compared with those of breastfed infants. During this period, Lactobacillus, Staphylococcus, Megasphaera, and Actinobacteria are more abundant in breastfed infants, whereas the genera of Clostridiales and Proteobacteria are more abundant in formulafed infants [58]. It has been found that the abundance of beneficial Bifidobacterium, Lactobacillus, and Clostridium is positively correlated with breast feeding and negatively correlated with formula feeding, and the abundance of functional Lachnospiraceae was found to be decreased in breastfed infants [68,69]. In addition, the intestinal microbiome of most breastfed infants showed an increased ability to synthesize methionine, branched-chain amino acids, cysteine/serine, threonine, and arginine [69]. Studies have shown that the intestinal microbiota of infants that were exclusively breastfed (EBF) before the introduction of solid foods was quite different from that of non-EBF infants, which was characterized by higher abundance of Bifidobacterium and lower abundance of Bacteroidetes and Clostridiales [70]. Besides breast feeding, the intake of solid food led to dramatic changes in microbial composition, not only in terms of a transition to relatively stable communities, but also in an increase in SCFA production, vitamin biosynthesis, and carbohydrate synthesis [28]. Interestingly, the patterns of supplementary food intake also affect the intestinal microbiota. The bacterial α-diversity and Roseburia abundance of infants were lower in a 12-month-old baby-led introduction to solids group (i.e., an improved version of baby-led weaning, in which solid foods are introduced at six months of age and then self-feed the family food) than those in a traditional intake style group (i.e., parents used a spoon to feed mushy food) [71].
《3.3. Environmental factors》
3.3. Environmental factors
Environmental factors, especially early-life antibiotic use, can delay the maturation of the intestinal microbiota. For example, children exposed to antibiotics showed a slower maturity than those that were not exposed, which was most obvious between the 6th and 12th months and was mainly due to the lack of Lachnospiraceae and Erysipelotrichaceae [58]. Furthermore, early pulsed antibiotic treatment (PAT) with a β-lactam or macrolide could impact the metabolism of the host intestinal microbiota; the effects included reducing the glycolysis and gluconeogenesis pathways and increasing the citric acid cycle, nucleoside synthesis, and amino acid synthesis [72]. In addition, in PAT with low-dose penicillin-treated mice, the abundance of Lactobacillus, Candidatus Arthromitus, and Allobaculum decreased, and immune-related gene expression was altered, including decreasing differentiation, activation, adhesion, recruitment, and quantity of immune cells; these effects were found to lead to obesity and to continue to affect the host throughout its life [73]. Furthermore, the alterations induced by early antibiotic exposure predisposed the host to diseases, such as obesity [74,75], T1D [76], and asthma [77].
《4. Effects of the intestinal microbiota on intestinal homeostasis and neurodevelopment》
4. Effects of the intestinal microbiota on intestinal homeostasis and neurodevelopment
《4.1. The mechanism of the microbiota and its metabolites on intestinal development and homeostasis》
4.1. The mechanism of the microbiota and its metabolites on intestinal development and homeostasis
The intestinal microbiota is a crucial factor for human health and well-beings. First, gut microbiota promote intestinal maturation [78]. The structure and function of the GI tract in newborns are immature [79]. Gut microbiota play a vital role in the development of the immune system—especially in intestinal homeostasis— by modulating the nutrient metabolism, epithelial barrier integrity, and immunity [79–81]. A variety of intestinal bacteria and gut-derived metabolites participate in maintaining intestinal homeostasis, including resistance to foreign pathogen invasion and anti-inflammatory homeostasis (Fig. 1). Among the numerous commensal microbiota, segmented filamentous bacteria (SFB) are a particularly important intestinal microbe in regulating the intestinal homeostatic immune response [82,83]. T and B cells are the major cellular components of the adaptive immune response. SFB attaches to the epithelial cells of the digestive tract tightly, initiates the activation of T and B cells, which contributes to the homeostatic immune response [82,84,85].
《Fig. 1》

Fig. 1. The role of the intestinal microbiota in immunity development and homeostasis maintenance. The intestinal microbiota promotes the development and maturation of the adaptive immune system and helps the host to achieve a balance between resistance to foreign pathogen invasion and anti-inflammatory homeostasis. SFB: segmented filamentous bacteria; GPR: G-protein-coupled receptor; TGF-β: transforming growth factor-β; MMP: matrix metalloproteinase; DC: dendritic cells; SAA: serum amyloid A; ROS: reactive oxygen species; HDAC: histone deacetylase; IL: interleukin; Treg: regulatory T; Foxp3+ : forkhead box protein 3; Th: T helper; IgA: immunoglobulin A.
T helper (Th) cells are a type of effector T cell that are tightly regulated by the gut. Early colonization of intestinal microbiota leads to the differentiation and responses of immune Th cell subsets, such as Th1, Th17, and regulatory T (Treg) cells [80,84], which are critical in maintaining intestinal immune homeostasis. For example, SFB colonization facilitates the induction of Th17 cells by inducing serum amyloid A (SAA; an acute-phase plasma protein, which is synthesized in the liver) [86] and reactive oxygen species (ROS; short-lived electrophilic chemicals produced by superoxide and incomplete reduction) [87] from intestinal epithelial cells [88]. Matured Th17 cells protect the host from bacterial infection by secreting interleukin (IL)-17, IL-17F, and IL-22, especially in the lamina propria of the small intestine [85]. Besides SFB, the development of forkhead box protein 3 (Foxp3+ ) Treg cells can be induced by Clostridium [89]. The Clostridium species activates the production of matrix metalloproteinases (MMPs), which generate biologically active transforming growth factor-β (TGF-β), leading to the development and maintenance of Foxp3+ Treg cells [90]. Foxp3+ Treg cells can secrete IL-10 after being activated [91]. IL10 inhibits the differentiation of Th1 and Th17 by restraining IL12 and IL-23, and then drives macrophages to exert immune tolerance in order to maintain immune homeostasis [91].
The germinal center in Peyer’s patches of the small intestine can be activated by the attachment of SFB to promote B cell activation [92]. Activated B cells (i.e., plasma cells) can migrate back to the intestinal mucosa through the hemolymphatic cycle; the circulating plasma cells then secrete immunoglobulin A (IgA, the main antibody isotope produced on the mucosal surface, which assists in the development and maintenance of the intestinal microbiota) [93]. Moreover, IgA secretion can prevent the adhesion of SFB to epithelial cells, thereby inhibiting immune activation [82].
In addition to directly interacting with the host, symbiotic microbiota can regulate the immune system through intestinalderived metabolites, such as SCFAs, including butyrate, acetate, and propionate [94,95]. SCFAs promote the development of intestinal immunity through G-protein-coupled receptors (GPRs) [80,90,96,97]. For example, the development of Th1 and Th17 cells is triggered by acetate via inhibiting histone deacetylase (HDAC) activity [98]. Acetate can maintain the integrity of the intestinal epithelial barrier by inhibiting the transport of Escherichia coli O157:H7 Shiga toxin, inducing ROS production, and promoting the development of Th17 cells to protect the host from fatal infections [99]. Moreover, Foxp3+ Treg cell differentiation can be promoted by butyrate via stimulating GPR109A signal transduction. The accumulation of Foxp3+ Treg cells and their suppressive activity can be motivated by butyrate and propionate by inhibiting HDAC activity through GPR43 [89,90]. Thus, the intestinal microbiota promotes the development and maturation of the adaptive immune system and helps the host achieve a balance between resistance to foreign pathogenic invasion and anti-inflammatory homeostasis.
Gut-derived LPSs are another important factor in the development of the innate immune system. LPSs are considered to be one of the most effective stimulators of the host’s innate immune system [100]. For example, researchers have demonstrated that LPSs may affect the maturation of Treg cells in gut-associated lymphoid tissue [101–103]. In addition, LPSs can be transported to the subepithelial dome by the microfold cells on the luminal surface of Payer’s patches, where they are sampled by dendritic cells and presented to lymphocytes, which can lead to the maturation of IgAproducing B cells [104,105]. Furthermore, a predominance of low-endotoxin LPSs may induce and change the activation of the innate immune system, induce Treg cells, or prevent the Th1/ Th17 response, all of which are essential for regulating the balance of intestinal immunity [101]. Thus, LPSs are crucial for intestinal immune cells.
《4.2. The intestinal microbiota and the CNS during neurodevelopment》
4.2. The intestinal microbiota and the CNS during neurodevelopment
The ability of gut microbes to influence neurodevelopment causes them to have another major effect on human development. Such findings have been demonstrated by the behavior of and cognitive assessments in young germ-free (GF) animals [106]. It is known that the hippocampus, striatum, and amygdala regions of the brain are related to learning, memory, exercise, and emotion (Fig. 2). The hippocampus is mainly responsible for memory and spatial navigation. It was found that fructooligosaccharide (FOS) activity, 5-hydroxytryptamine receptor 1A (5-HT1A) levels, and the expression of brain-derived neurotrophic factor (BDNF) were reduced in the hippocampus of GF mice, resulting in the impairment of their working memory [107,108]. The striatum integrates the motor and emotional responses and is closely related to the motor-related basal ganglia and limbic systems [109]. Increased dopamine, serotonin neurotransmitters, and synaptic vesicle proteins (an indirect marker of synaptic genesis) in the striatum of GF mice result in anxiety-like behavior [110]. Moreover, decreased levels of N-methyl-D-aspartic receptor (NMDAR), 5- hydroxytryptamine receptor 1 (5-HT1), and BDNF in the amygdala, part of the ‘‘emotional brain” limbic system, together with alterations of striatum in GF mice, lead to an increase in risk-taking behavior [107,111].
《Fig. 2》
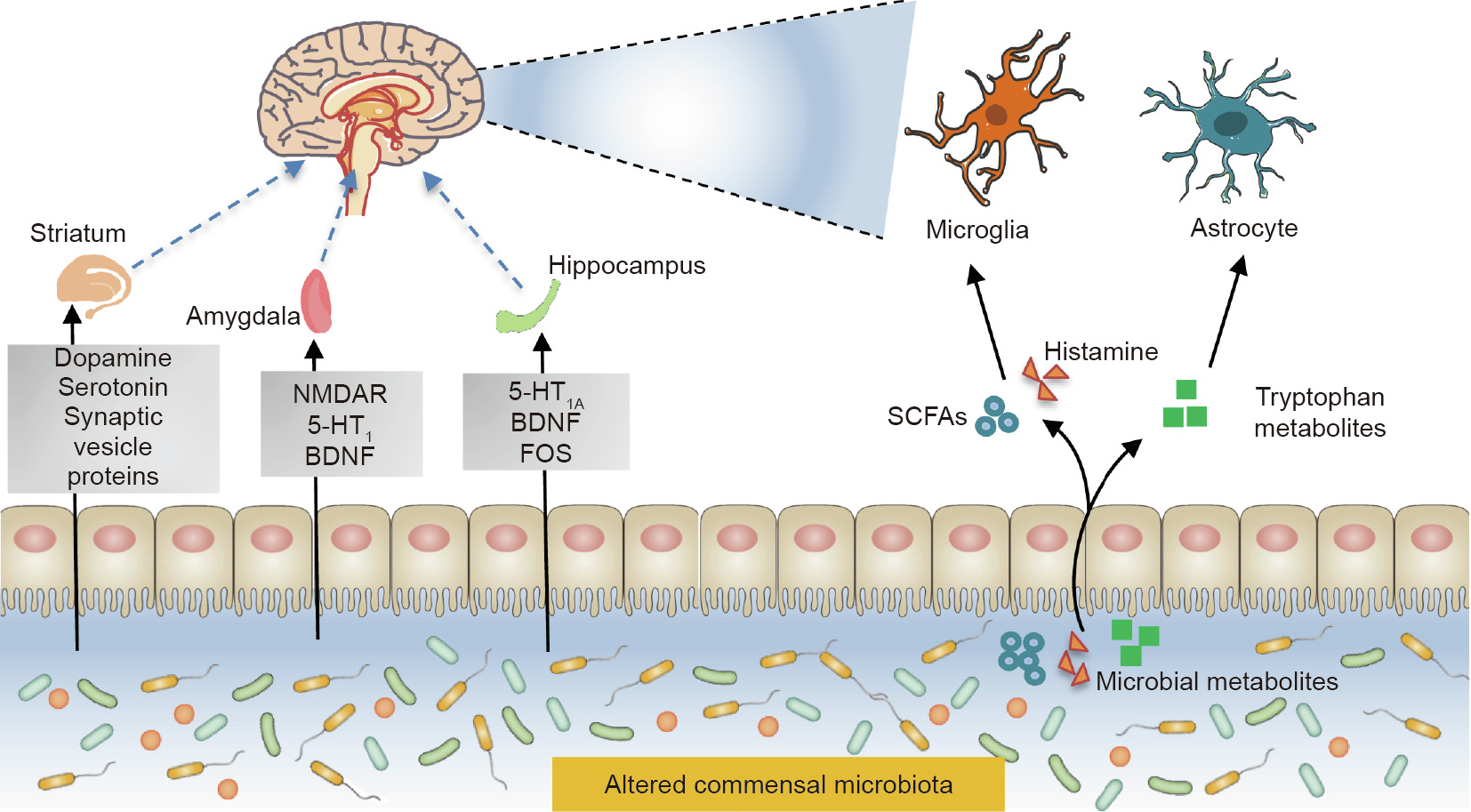
Fig. 2. Neuroimmune crosstalk between the intestinal microbiota and the CNS during neurodevelopment. NMDAR: N-methyl-D-aspartic receptor; 5-HT1A: 5- hydroxytryptamine receptor 1A; 5-HT1: 5-hydroxytryptamine receptor 1; BDNF: brain-derived neurotrophic factor; FOS: fructooligosaccharide.
The exact mechanisms of the microbiota–gut–brain axis—by which the brain and gut perform bidirectional communication— are not fully understood, although it is known that they involve neural, hormonal, and immunological signaling [112]. Two main types of nerve cells participate in the crosstalk between the gut microbiota and the CNS [113,114]. One of these nerve cell types is the microglia, which are the most abundant innate immune cells in the CNS, accounting for 10%–15% of the glial cells in the brain [36]. The microglia are involved in the development of the CNS in early life and participate in antigen presentation, phagocytosis, and inflammation regulation throughout a human’s life [115,116]. Microbial metabolites, including SCFAs, histamine, and tryptophan metabolites, are essential messengers in the axis [97,117–119]. SCFAs play an important role in promoting maturation and maintaining the normal physiological functions of the microglia [40]. Histamine is another important developmental signal for the microglia, as it has been reported to regulate host behavior and cognition [120,121]. Aside from food-derived [122], histamine-secreting bacteria from the gut also include Escherichia coli, Lactobacillus vaginalis, and Morganella morganii [123]. Microbial-derived histamine impacts the activation of the microglia and the secretion of pro-inflammatory factors from the microglia, which contribute to immune homeostasis during neurodevelopment [124].
Astrocytes, a group of glial cells with diverse functions, are another important immune cell type in the CNS. In addition to playing a prominent role in neuroinflammation, astrocytes are involved in ion homeostasis, neurotransmitter clearance, glycogen storage, maintenance of the blood–brain barrier, and nerve signal transduction [125]. Most undigested dietary tryptophan in the gut lumen is converted to indole, which can then be metabolized or modified further by microbial and hepatic enzymes, producing indole derivatives of varying affinities for aryl hydrocarbon receptors (AhRs) [36]. Microbial metabolites from dietary tryptophan can activate AhRs to attenuate inflammation in astrocytes. Moreover, 5-hydroxytryptamine (5-HT), which is derived from tryptophan via the formation of 5-hydroxytryptopha, is known to act as the key neurotransmitter in regulating neuronal differentiation and migration, as well as axonal growth, myelination, and synapse formation during CNS development [126,127]. It has also been shown that intestinal microbiota clostridial species play an important role in regulating the 5-HT level derived from enterochromaffin [118]. Recently, alteration of intestinal microbiota in the autism spectrum disorder (ASD) mouse model was found to correlate with impaired production of intestinal 5-HT [128]. Interestingly, mice fed a nutrient-rich diet had significantly improved microbial diversity compared with mice fed a normal diet; accompanied by the apparent alteration of the gut microbiome, the nutrient-rich diet-fed mice exhibited improved memory, learning, and anxiety behavior [129]. This evidence indicates that dietinduced transient changes in the microbiome can affect behavior, which is an attractive issue that requires further investigation.
《5. Clinical implications and the gut microbiota from infancy to childhood》
5. Clinical implications and the gut microbiota from infancy to childhood
An understanding of the characteristics of a healthy intestinal microbiota from infancy to childhood could provide novel therapeutic targets and permit the early detection of infants susceptible to disease. It could also contribute new strategies to prevent and treat these diseases, including intervention in the microbiota ‘‘window” of early life [130]. Numerous studies have shown that certain microbial signatures are associated with an increased risk of pediatric diseases and even adult diseases.
《5.1. Obesity》
5.1. Obesity
In the last decades, the prevalence of overweight and obese children continues to rise in most countries and is considered to be a major global health challenge [131]. Recent reports estimate that 40 million children under the age of five and over 330 million children and adolescents aged 5–19 were obese in 2016 [132]. Obesity, which usually begins in childhood or adolescence, is one of the greatest risk factors for many major chronic diseases, such as type 2 diabetes and coronary heart diseases [133]. Interestingly, repeated exposure to antibiotics in early childhood is related to a higher average body mass index (BMI) and a propensity toward obesity [75]. Along with the genetic components of the human genome, the human microbiome plays an important role in the development of obesity [6]. A recent finding showed that the meconium microbiome of three-year-old overweight children differed from that of normal-weight children, in that it had a higher proportion of Bacteroidetes [134]. The same study presented an association between the intestinal microbiota and infants being overweight during pregnancy and birth (the first-pass meconium). A study on Belgian children showed that obese children had a higher proportion of Firmicutes/Bacteroidetes, as well as higher levels of Lactobacillus, and that the proportion of Staphylococcus aureus was positively correlated with inflammatory markers and energy intake, respectively [135]. Moreover, in the first few months of life, colonization of Streptococci and Bifidobacterium can predict changes in BMI, and the effects on BMI depend on future antibiotic use [136]. Notably, Stanislawski et al. [137] confirmed that the intestinal microbiome classification and alpha diversity measurement of two-year-old infants had the strong association with BMI in later life, which could be an indicator for the identification of high-risk children. More recently, depletion of Blautia species (especially Blautia luti and Blautia wexlerae) was found in obese children, and could result in metabolic inflammation and insulin resistance [138].
Altered intestinal microbiota may contribute to the development of obesity through metabolites such as SCFAs. SCFAs are ligands that can bind to GPR41 and GPR43 in the enteroendocrine L cells of the ileum and colon as well as in adipocytes and immune cells [139,140]. The role of SCFAs in the development of obesity is controversial. Studies have shown that a diet rich in fiber is associated with a lower incidence rate of obesity and metabolic syndrome [141]. This may be due to the fact that SCFAs, which are converted from fermentable dietary fiber, can prevent obesity by activating free fatty acid receptors (FFARs), promoting the release of peptide YY (PYY) and glucagon-like peptide-1 (GLP-1), which have anorexigenic effects and can respectively increase satiety and prevent obesity [140]. In direct contrast, however, other studies have indicated that diets rich in fiber may actually promote the development of obesity rather than preventing it. For example, obese children have a high abundance of Firmicutes/Bacteroidetes, which promote the binding of SCFAs and GPR41/GPR43 [142]; the activation of GPRs in adipocytes then triggers the secretion of PYY in intestinal endocrine cells [143]. In turn, PYY can slow down the intestinal transit time, increase the energy extraction efficiency, and lead to obesity [144]. Other findings have also shown that an excess of SCFAs can disturb the balance of energy regulation, and can simultaneously interact with FFAR and participate in pancreatic β-cell glucose-stimulated insulin secretion and the release of appetite-controlling peptide hormones [145]. The role of SCFAs in obesity and related diseases thus remains an important issue.
LPSs produced by Gram-negative bacteria are another important factor in obesity [146]. In lipogenesis, LPSs are involved in the immune response; they mediate the inflammation and infiltration of immune cells, thereby disturbing the intestinal barrier and the subsequent translocation of bacteria or bacterial products [142]. In addition, alteration of the intestinal microbiome can lead to changes in bile acid pools in the liver, heart, and kidney, which then affect farnesoid X receptor nuclear antagonists, leading to obesity and insulin resistance [147].
《5.2. Type 1 diabetes》
5.2. Type 1 diabetes
T1D is an autoimmune disease that targets the pancreatic islet β cells [148]. Over time, it has become a major public health concern around the world [15]. According to the International Diabetes Federation, 1 110 100 children and adolescents under the age of 20 have T1D, and the global overall annual increase in T1D is around 3% [149]. It has been shown that T1D is associated with autoantibodies, which can be found starting from the first year after birth, and that those with T1D at a young age have a more serious condition [150]. Since the incidence of individuals carrying T1D-associated human leukocyte antigen risk alleles is only 50%, non-genetic factors are important in the development of T1D [7].
Recent studies have emphasized the role of the intestinal microbiota in T1D. Leiva-Gea et al. [151] found that the intestinal microbiota, including Bacteroides, Ruminococcus, Veillonella, Blautia, and Streptococcus genera, were enriched in T1D patients, and the expression of metabolic genes related to lipid and amino acid metabolism, ATP-binding cassette transport, LPS biosynthesis, arachidonic acid metabolism, antigen processing and presentation, and chemokine signaling pathways was enhanced. Moreover, Vatanen et al. [148] found lower levels of genes involved in bacterial fermentation and SCFAs biosynthesis in T1D patients. They also found that healthy children may have a large amount of probiotic bacteria, such as Lactobacillus, whereas the microbiota of children with T1D may be dominated by Bacteroides [148]. Notably, a reduction in intestinal microbial metabolite SCFAs is strongly associated with susceptibility to diabetes [152,153]. Due to the influence of diet, infection, medications, and other factors, a decrease in fecal SCFAs, especially acetate and butyrate, leads to a decrease of Treg cells in the islets and an increase of autoimmune T cells, and finally induces the occurrence of T1D [154,155]. To be more specific, a lack of butyrate reduces the attachment of intestinal epithelial cells, increases intestinal permeability, and increases bacterial antigen exposure, which induces an immune response that leads to autoimmunity in T1D [156]. Aside from SCFAs, a microbiota that leaves the host prone to T1D was demonstrated in early PATtreated mice, characterized by decreased Bacteroidetes and Actinobacteria and increased Proteobacteria and Akkermansia muciniphila [76].
《5.3. Allergic diseases: Asthma and food allergies》
5.3. Allergic diseases: Asthma and food allergies
Asthma is a chronic inflammatory disease that affects more than 300 million people worldwide [157]. Food allergies are common in infants and young children [158], with an overall prevalence among children of 12% [130]. The microbiota hypothesis suggests that the intestinal microbiota links environmental changes with the immune system [159] in immune diseases such as allergies and asthma [32].
A variety of studies have examined the relationship between the intestinal microbiota and allergic diseases in children. Zimmermann et al. [160] used a meta-analysis to determine that the incidence of allergies and asthma in children was closely related to the enrichment of Bacteroidaceae, Clostridiaceae, and Enterobacteriaceae, and the depletion of Bifidobacteriaceae and Lactobacillaceae. Another study of Canadian infants found that a low abundance of Faecalibacterium, Lachnospira, Veillonella, and Rothia was associated with a high risk of allergy and asthma in three-month-old infants [157]. Similarly, a longitudinal study monitoring infants 1–11 months old in the United States showed that one-month-old infants who had a lower abundance of Bifidobacterium, Lactobacillus, Akkermansia, and Faecalibacterium genus would have a higher incidence of asthma at four years of age [161]. Furthermore, a long-lasting alteration in the microbiota was found in infants with macrolide use, which was associated with an increased risk of asthma and a predisposition to antibioticassociated weight gain. This finding highlights the contribution of the early-life intestinal microbiome to health [77]. In addition, microbial metabolites, such as a high concentration of 12,13- diHOME, can impact the incidence of allergies [162].
Cow’s milk allergy (CMA) is one of the most common food allergies in infancy, affecting 2%–3% of infants worldwide [163]. Studies have showed a close relationship between CMA and the intestinal microbiota. For example, Berni Canani et al. [164] found that infants with CMA had a diversified intestinal microbiota, which was mainly characterized by the enrichment of Lachnospiraceae and Ruminococcaceae and the reduction of Bifidobacterium and Escherichia. Bunyavanich et al. [165] also conducted a longitudinal study on infants with milk allergies in which they analyzed stool samples from infants 3–16 months old, and then performed clinical evaluations, milk-specific IgE levels, and milk skin prick tests at several timepoints (at enrollment, 6 months, 12 months, and then once a year until the age of eight). Interestingly, the infants enriched with Clostridium and Firmicutes were associated with milk allergy symptoms that subsided by the time they reached eight years of age. Notably, it was found that while most early childhood food allergies will self-heal in later childhood, a sensitivity to certain allergens (e.g., peanuts or nuts) may persist into adulthood [130].
Based on the different underlying pathophysiologies, adverse food reactions can be divided into food intolerance (nonimmune-mediated) and food sensitivity (immunizationmediated) [166]. Aside from the immune mechanism, existing research has linked food allergies to various microbial signals that may be caused by intestinal infections or by changes in the commensal gut microbiota [167,168]. For example, Bifidobacterium promotes intestinal epithelial integrity and prevents lethal infection by producing acetate, and then alleviates food allergies by inducing mast cell apoptosis [99]. Also, SCFAs or tryptophanderived metabolites can directly regulate the mucosal immune function and intestinal barrier integrity, thereby affecting the body’s susceptibility to food sensitivity [169,170]. Furthermore, butyrate regulates the host’s tolerance to food antigens or allergens by adjusting the ratio and function of transcription factor Foxp3+ Treg cells [171,172]. Interestingly, the presence of butyrate can lead to food allergies, while the absence of bacterial metabolites may impair intestinal homeostasis, and then leads hosts to be susceptible to food allergies [173].
《5.4. Stunting》
5.4. Stunting
Stunting is a severe growth-impairment disease that affects 155 million children under the age of five around the world [174]. Stunting is associated with repeated diarrheal infections, poor sanitation, and nutritional deficiencies, and the latter are related to an altered intestinal microbiota [175]. Importantly, stunted children exhibit a great relative abundance of Escherichia coli/Shigella sp., Campylobacter sp., and Proteobacteria, but a reduction in Clostridia [45,176]. In another study on south India children, stunted children were found to have a rich abundance of Bacteroidetes phylum, Campylobacterales order, and Desulfovibrio genus [177].
The cause of stunting is probably environmental enteric dysfunction (EED) [178,179]. It is also hypothesized that small intestinal bacterial overgrowth characterized by oropharyngeal species contributes to EED, as such overgrowth can cause local inflammation that results in impaired absorptive and digestive functions of the gut [177]. Disorders of the intestinal microbiota in stunting have been linked with changes in immune responses [180,181] and intestinal permeability [180,182,183]. Microbial products with a specific biology and function may affect the sensitivity of children to EED. For example, Citrobacter rodentium was found to reduce both signs of EED in Zambian children—namely, intestinal villus height and increased intestinal permeability [184]. The microbiota in stunted children can reduce some metabolic pathways such as those for amino acids, carbohydrate utilization, and B-vitamin metabolism, which then leads to EED [185].
《5.5. Autism spectrum disorder》
5.5. Autism spectrum disorder
ASD is a neurodevelopmental disorder that occurs in the first three years after birth and is characterized by social communication disorders, limited and narrow interest, and repetitive behavior [186]. ASD affects 2.24% of children in the United States [187], and the prevalence of ASD is 11.8 per 10 000 children in China [188]. In addition to cognitive aspects, GI disorder is a common nonneurological symptom in children with ASD [189].
In a report on Chinese children with ASD, an enriched proportion of phylum Bacteroidetes/Firmicutes, a relative abundance of genus Sutterella, Odoribacter, and Butyricimonas, and reduced Veillonella and Streptococcus were found [190]. Furthermore, certain intestinal microbiota may be involved in the pathogenesis of ASD. For example, a higher prevalence of Sutterella species were found in biopsies of the GI tract of ASD children with GI disturbances, compared with healthy controls [191]. In addition, Sandler et al. [192] found that treating ASD children with six weeks of the oral antibiotic vancomycin, an antibiotic against Clostridia, significantly improved both neurobehavioral symptoms and GI symptoms in eight out of ten children. These studies strongly suggest that bacteria such as Sutterella and Clostridia play a role in the pathogenesis of autism.
The microbiota might also be involved in the immune and inflammation responses of ASD. For example, microglia dysregulation has been reported in ASD [193], whose homeostasis, maturation, and function are affected by intestinal microbiota such as Bacteroides distasonis, Lactobacillus salivarius, and Clostridium cluster XIV [40]. In addition, microbial metabolite SCFAs can inhibit the maturation of monocytes, macrophages, and dendritic cells by inhibiting HDAC, and then alter their ability to capture antigens and reduce the production of pro-inflammatory cytokines [194]. SCFAs can also regulate the secretion of hormones in the gut, including PYY, GLP-1, insulin, ghrelin, and leptin, all of which have been demonstrated to be involved in ASD [195].
Changes in aromatics, glutamate metabolism, and bile acid metabolism in the intestinal microenvironment of children with ASD are also extremely important. For example, in an ASD mouse model, the reduction of intestinal Bifidobacterium and Blautia was associated with a lack of bile acid and tryptophan metabolism as well as impaired social interactions [128]. In addition, Wang et al. [187] found that changes in the glutamate metabolism of ASD patients were related to a low level of Bacteroides vulgatus and a high level of harmful Eggerthella lenta and Clostridium botulinum. The scholars also identified reduced intestinal 2-keto-glutamate as a potential biomarker of ASD; it is directly related to gut hormone 11-deoxy human prostaglandin F (PGF2) and affects the neurotransmitter glutamate inhibition/excitatory imbalances [187].
《5.6. Inflammatory bowel disease》
5.6. Inflammatory bowel disease
The global incidence of pediatric IBD has been rising in recent years [196]. In the United States and Canada, the incidence is about ten cases per 100 000 children and continues to rise [196,197]. Among pediatric IBD cases, 4% appear before the age of five, 18% before the age of ten, and the peak in adolescence [197]. IBD, including Crohn’s disease (CD) and ulcerative colitis (UC), usually begins in childhood or adolescence, and is characterized by chronic intestinal inflammation due to complex interactions of genetic determinants, disruption of mucosal barriers, aberrant inflammatory signals, loss of tolerance, and environmental triggers [198,199].
Among the environmental risk factors, the intestinal microbiota plays an important role in the pathogenesis of pediatric IBD. The microbial diversity of children with pediatric IBD is significantly reduced [200]. In UC, the abundance of known positive bacteria in the intestinal tract, such as Eubacterium rectale and Faecalibacterium prausnitzii, has been found to be significantly reduced, whereas known pathogens such as Escherichia coli are enriched [200]. CD is associated with reduced aα-diversity; an increased abundance of Enterobacteriaceae, Pasteurellaceae, Veillonellaceae, and Fusobacteriaceae; and a decreased abundance of Erysipelotrichales, Bacteroidales, and Clostridiales [201]. Another study using metaproteomics reported four increased phyla in pediatric IBD: Proteobacteria, Verrucomicrobia, Ascomycota, and Spirochetes [202]. The genera most obviously related to pediatric IBD were the decreased Bacteroides and the increased Faecalibacterium [202].
Gut-derived metabolites are also involved in the development of pediatric IBD [200,203–206]. For example, the number of SCFA-producing bacteria and the concentration of butyrate have been shown to be reduced in pediatric IBD, which are related to the significantly increased number of pro-inflammatory immune cells in the intestinal mucosa, and are even linked with the appearance and severity of IBD [203].
《6. Microecological intervention: Probiotics/prebiotics and fecal microbiota transplantation》
6. Microecological intervention: Probiotics/prebiotics and fecal microbiota transplantation
《6.1. Probiotics/prebiotics: Treatment of pediatric diseases and safety issues》
6.1. Probiotics/prebiotics: Treatment of pediatric diseases and safety issues
Interventions of the intestinal microbiota by providing probiotics or prebiotics can effectively improve the symptoms of these metabolic-, allergic-, and auto-immune-disorder-related pediatric diseases [206–209]. Probiotics and prebiotics are microbiotamanagement tools that benefit host health. The consensus definition of probiotics is ‘‘live microorganisms, when administered in adequate amounts, confer a health benefit on the host,” and that of prebiotics is ‘‘a substrate that is selectively utilized by host microorganisms, conferring a health benefit” [24,210,211].
Multiple studies have investigated the effect of prebiotics on pediatric diseases. Nicolucci et al. [206] observed that inulin treatment significantly reduced the body fat and trunk fat of obese children, and was associated with increased Bifidobacterium and decreased Bacteroides vulgatus. In a mice model, long-chain inulin was shown to inhibit T1D by regulating gut-pancreatic immunity, barrier function, and microbial homeostasis [21]. Moreover, prebiotic Bimuno® galactooligosaccharides were used to treat children with autism for six weeks, and resulted in improved GI function and antisocial behavior, a significant increase in Lachnospiraceae family, and distinct changes in fecal and urine metabolites [207]. A recent study found that there was a prominent remission in the severity of autism and GI symptoms in children with ASD who were given mixed probiotics of four probiotic strains (Bifidobacterium infantis Bi-26, Lactobacillus rhamnosus HN001, Bifidobacterium lactis BL-04, and Lactobacillus paracasei LPC-37) with FOS [212]. When given a probiotic supplement of Lactobacillus acidophilus, stunted children in India exhibited improved weight, height, and morbidity profile with respect to diarrhea, fever, cough, and cold [213].
The efficacy of probiotics for autoimmune diseases is a hot topic, albeit a controversial one. For example, Savilahti et al. [208] provided pregnant women (where one or both of the couple had a doctor-diagnosed allergy) with a mixed probiotic capsule of Lactobacillus GG (ATCC 53103), Lactobacillus rhamnosus LC705 (DSM 7061), Bifidobacterium breve Bb99 (DSM 13692), and Propionibacterium freudenreichii ssp. shermanii JS (DSM 7076), from the 36th gestational week until delivery. The babies were given the same capsule for six months, and were followed up at the ages of 2, 3, and 15 years. However, the probiotic treatment had no effect on the occurrence of autoimmune diseases [208]. In contrast, Huang et al. [23] evaluated the effects of Lactobacillus paracasei (LP), Lactobacillus fermentum (LF), and combinations thereof (LP + LF) in children with asthma, and found that probiotics intervention effectively reduced the severity of asthma and that combinations of LP plus LF seemed to be more effective than LP or LF alone. In addition, Buffington et al. [209] showed that probiotic Lactobacillus reuteri corrected oxytocin levels and synaptic dysfunction in the ventral tegmental area of the brain in maternal high-fat diet-induced obese mice and selectively reversed social deficits—namely, specific behavioral abnormalities associated with neurodevelopmental disorders in the offspring of obese mice.
The development of sepsis is one of the risks of probiotics supplementation, especially in newborns and pregnant women [214]. For example, sepsis by Lactobacillus rhamnosus GG has been reported in infants, including one case with complicated postoperative period after the repair of double-outlet right ventricular and pulmonary artery stenosis and two cases with short bowel syndrome [215,216]. There have also been reports of increased allergic reactions in infants after taking Lactobacillus [217–219]. As for deleterious metabolic activities, an increasing trend in infection complications was observed in children in intensive care using probiotics [220]. Other metabolic concerns include the influence of D-lactic acid produced by the probiotic and the deconjugation of bile salts [221]. Although it is theoretically possible to perform lateral gene transfer between probiotic organisms and other organisms in the intestine or elsewhere, clinical evidence of antibiotic resistance transfer has not been reported thus far [214,222]. To improve the safety of probiotic interventions, research on the underlying mechanisms of probiotic activity is urgently needed. The theoretical risks of probiotics must also be evaluated according to the characteristics of the microorganisms used. Furthermore, organoids engineered with microbiota niches and related animal models should be reasonably used to better explore the mechanisms of probiotics.
《6.2. Fecal microbiota transplantation: Treatment of pediatric diseases and safety issues》
6.2. Fecal microbiota transplantation: Treatment of pediatric diseases and safety issues
Fecal microbiota transplantation (FMT) is a well-tolerated, simple, and promising treatment for pediatric diseases. FMT has been performed to treat pediatric diseases such as ASD [223], IBD [224], Clostridium difficile infection [225], and refractory diarrhea [226]. In line with findings in adults, the symptoms of two-year-old children with Clostridium difficile infection were relieved by FMT and showed no recurrence within six months of follow-up [225]. Two children with refractory diarrhea were also relieved with FMT [226]. Interestingly, after being given an initial high dose of FMT followed by a daily low-maintenance dose for 7–8 weeks, GI symptoms in children with ASD aged 7–16 years were reduced by approximately 80%, and the improvement lasted for eight weeks [223]. Positive effects of FMT have also been found in IBD: Two weeks of FMT treatment in children aged 10–17 years resulted in improved UC and CD colitis scores, while the related side effects were self-limiting and benign [224]. In a recent study on maternal FMT, seven infants born by Cesarean section received FMT from their mothers; their microbial composition gradually showed similarities to that of infants from vaginal births [227]. This study indicates that the FMT of mothers after birth can restore the microbial community in babies born by Cesarean section, providing a new direction for preventing pediatric diseases. More recently, 30 infants born by Cesarean section were orally seeded with maternal vaginal bacterial samples; however, the relevant test results have not been released [228]. Nevertheless, some studies reported adverse effects [229]. Thus, standard protocols should be established to maintain FMT safety.
《7. Conclusions and perspectives on the gut microbiota of infants and children》
7. Conclusions and perspectives on the gut microbiota of infants and children
The gut microbiota is closely related to human growth and development. The colonization and flora of the microbiota in the first three years of human life is an extremely important ‘‘window” stage for the establishment of the microbiota and for possible medical intervention. However, the dynamic changes of the intestinal microbiome and its causal relationship with diseases in infants and young children need to be clarified in order to promote highly specialized treatment. Although the results from the existing research are based on long-term follow-up studies with a large sample size, the effect of these studies is limited by their singlefactor sample grouping design, as there are multiple influencing factors in intestinal microecology. Future studies with multiplefactor sample grouping designs are needed.
In addition to the gut bacteria discussed herein, intestinal viruses and fungi are important components of the human intestinal community [230,231]. A report showed that the intestinal fungi of human infants aged 1–4 months were mainly surrounded by Saccharomycetales and Malasseziales; fungi eventually matured within 5–11 months until the presence of Malasseziales was reduced while Saccharomycetales was retained [161]. Recent studies have demonstrated that intestinal fungi contribute to ASD [232], asthma [233], allergy [234], obesity [235], and T1D [236]. A few studies have provided preliminary evidence that eukaryotic viruses have the ability to promote intestinal homeostasis and shape mucosal immunity [237]. For example, certain enteroviruses can infect β cells in vitro and have been detected in the islets of T1D patients, by the mechanism of which enteroviruses are related to T1D [238–240].
Recently, a debate has been raised about whether there is a microbiome in the placenta and what the involvement of the placental microbiome might be in shaping the early-life microbiota after birth. Early studies have reported that the microbiota of the placenta during healthy term delivery is rich in Lactobacillus sp., Propionibacterium sp., and members of the Enterobacteriaceae family [241]. Another study found that the placenta collected during sterile Cesarean sections was characterized by Proteobacteria [242]. Interestingly, oral bacteria are considered to be the main source of placental bacteria [243], which has been demonstrated in animal experiments [244,245]. On the other hand, recent studies have found that there is insufficient evidence to support the presence of bacteria in placental samples. De Goffau et al. [246] found that almost all the signals in 16S ribosomal RNA (rRNA) gene amplicon sequencing were related to the acquisition of bacteria during delivery or to the contamination of bacterial DNA in laboratory reagents, rather than having the placenta itself as their source; the only exception was Streptococcus agalactiae (group B Strepto-coccus). Also, about 5% of the samples collected before the onset of labor have detected no contamination signals. Similarly, the presence of bacteria cannot be detected from the placenta of term deliveries or spontaneous preterm births [247]. Nevertheless, it remains unknown whether there are bacteria in the placenta, so there is a need for follow-up observations of mothers and offspring.
Studies have shown that intervention in the gut microbiota can improve pediatric diseases including obesity, T1D, allergic diseases, ASD, and stunting. First, probiotic and prebiotic interventions show promising effects against these diseases (Fig. 3). Other microecological interventions also exist, such as postbiotics [248], phage treatment [249], FMT [223], and nutritional intervention [250]. In the development of new and effective microecological preparations, new ideas can arise and be proposed using mathematical modeling in order to promote the development of effective probiotics and artificial FMT strains [251].
《Fig. 3》

Fig. 3. Intestinal microbiota-based therapeutic methods to treat pediatric disease. Recent research has demonstrated that modulation of the intestinal microbiota can improve pediatric diseases such as obesity, T1D, ASD, IBD, allergic diseases, and stunting. Intervention methods include the administration of probiotics or prebiotics, postbiotics, phages, and FMT.
When developing probiotic formulations, it is necessary to pay attention to safety and permanence issues, since controversial results have been published in previous reports [23,208,209]. These controversies may be due to differences in the specific microecological preparation, dose, clinical endpoint, or target population [24]. In addition, a systematic review of 384 randomized controlled trials evaluating probiotics, prebiotics, or synbiotics showed that the discussion of side effects in these reports is often lacking or inadequate, which may lead to the wrong medications being prescribed and subsequent adverse effects in subjects [252]. Moreover, most single-strain probiotics cannot permanently alter the gut community, and the effectiveness only persists with repeated use [253]. Importantly, the theoretical risks of probiotics include systemic infections, deleterious metabolic activities, excessive immune stimulation in susceptible individuals, gene transfer, and GI side effects, which have been exhibited in case reports, clinical trial findings, and experimental investigations [214,221]. Hence, it is urgent to clarify the proper components of probiotic formulations.
The causal relationships between the gut microbiota and diseases remain to be clarified; therefore, time and effort must be spent to elucidate these interaction mechanisms. Moreover, in clinical applications, individual differences should be taken into consideration in order to achieve individualized microecological intervention treatments. With the development of nextgeneration sequencing technology [254], multi-omics analysis (i.e., genomics, meta-transcriptomics, macroproteomics, and metabolomics) [255], and omics (i.e., culturomics) [256], the link between the intestinal microbiota and disease is becoming increasingly recognized. Meanwhile, new microbiome technologies have emerged, such as the development of fabricated microbial ecosystems (termed as EcoFABs), which are see-through contained models of microbial ecosystems, and the gut-on-a-chip, which is equipped with intestinal epithelial cells, fluid flow around the cells, and the ability to simulate intestinal peristalsis [257]. These technologies will contribute to future research on the complex microbiome mechanisms. Further studies should focus on the contribution of the early-life microbiota to complexities involving pathophysiology, complications, and quality of life, and should aim to improve the long-term outcomes associated with pediatric diseases.
《Acknowledgments》
Acknowledgments
This study was supported by the National Key Research and Development Program of China (2018YFA0903200), the National Natural Science Foundation of China (81790633 and 30901190), the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2019-I2M-5-045), and the Public Welfare Technology Research Program of Zhejiang Province (LGF18H310004).
We acknowledged Dr. Siqing Yue from the Zhejiang University of Technology and Honglei Weng from Heidelberg University for their helpful comments on this manuscript.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Hanying Lv, Lijiang Zhang, Yuqiu Han, Li Wu, and Baohong Wang declare that they have no conflict of interest or financial conflicts to disclose.
《Authors’ contributions》
Authors’ contributions
B. Wang, H. Lv, L. Zhang, Y. Han, and L. Wu wrote the manuscript; B. Wang revised the manuscript.













 京公网安备 11010502051620号
京公网安备 11010502051620号




