《1. Introduction》
1. Introduction
The purpose of cardiac surgery is to restore the injured heart to full function. For many congenital cardiac defects, appropriate repair is able to establish nearly normal cardiac function. One example of such repair is closing abnormal holes between cardiac chambers in young children, which usually restores normal function that persists for the lifetime of the child. However, the advent of surgery for ischemic heart disease has resulted in new challenges for cardiac surgeons. Elderly patients with diffuse atherosclerosis (artery hardening) frequently develop obstructions to the arteries supplying blood to the heart muscle, resulting in a heart attack (myocardial infarction). The resulting loss of contractile cardiac cells decreases the functional capacity of the heart. This initial damage is frequently complicated by progressive changes in the heart, leading to thinning of the damaged regions and cardiac chamber dilatation, which ultimately results in the progression of cardiac failure, the most frequent cause of cardiovascular death and disability worldwide. Cardiac surgeons frequently perform coronary bypass surgery to provide new blood flow beyond a severe coronary stenosis or occlusion, which relieves chest pain (angina), but does not necessarily restore cardiac function. As a result, the search for effective treatments to prevent or correct heart failure in patients who have suffered a heart attack (ischemic cardiomyopathy) has become the Holy Grail for cardiac surgeons.
Cardiac tissue engineering employs engineering concepts to modify the response of the injured heart in order to restore cardiac function. Although this definition of cardiac tissue engineering is not accepted by all investigators, it includes the essence of the restorative techniqures. In an editorial in the Journal of Thoracic and Cardiovascular Surgery, Dr. Vunjak-Novakovic [1] defined cardiac tissue engineering:
It is now almost 30 years since tissue engineering has been officially established, to offer ‘‘the application of principles and methods of engineering and life sciences toward fundamental understanding of structure–function relationships in normal and pathological mammalian tissues and the development of biological substitutes to restore, maintain, or improve tissue function.”
Restorative approaches frequently employ biodegradable biomaterials with the goal of modifying the healing of the infarct scar and the resulting cardiac tissue [1]. The mechanisms associated with the restoration of function include the stimulation of neoangiogenesis, matrix remodeling, and the recruitment of endogenous stem cells to modify tissue healing and restore function. Cardiac tissue engineering frequently employs transplantation of cells [2], the insertion of biomaterials [3], and/or the application of cytokines or cell products [4,5]. The restoration of cardiac function is dependent not only on the number of contractile elements, but also modification of ventricular volumes and the elastic characteristics of the injured heart wall [2–4].
Cardiac surgeons and heart researchers have developed a variety of tissue engineering approaches for ischemic cardiomyopathy. The goal of the preclinical studies was to evaluate novel potential therapies and then explore their clinical benefits in randomized clinical trials [6,7].
The field of cardiac tissue engineering is vast and beyond the scope of this commentary. Instead some examples of surgical cardiac tissue engineering of the injured heart from our previous research are presented below.
《2. Replacement of lost functional cardiomyocytes》
2. Replacement of lost functional cardiomyocytes
Our initial approach was to replace the cardiomyocytes lost following coronary occlusion with beating cardiomyocytes from newborn animals [8]. Cell implantation resulted in the engraftment of the beating cardiomyocytes in the infarct region, and cardiac function was temporarily improved. However, the engraftment was transient, and the beating cells did not beat synchronously along with the cardiac contraction. Most studies on stem cell transplantation into the heart have employed bone-marrow-derived stem cells. Although these cells do not provide new contractile elements, they have been shown to improve heart function in animal studies [2,4]; nevertheless, the benefits have been marginal in clinical trials [6,7]. Instead of contractile elements, the studies confirmed that bone-marrow-derived stem cells released various substances that stimulate endogenous repair mechanisms, resulting in new blood vessel formation (angiogenesis), scaffold repair (matrix repair), and the recruitment of bone-marrow-derived stem cells into the injured region [2]. The result was decreased thinning and dilatation of the infarct region and the prevention of progressive cardiac failure. The improvement in cardiac function was the result of changes in the environment of the cardiac damage. Unfortunately, cell transplantation was found to be less beneficial to patients in clinical trials than it was in preclinical animal studies. Recent studies have suggested that beating cardiomyocytes derived from human embryonic stem cells can improve heart function in a non-human primate model. However, the studies also showed that significant abnormal extra beats (arrhythmias) limited functional recovery [9].
《3. Cell transplantation to prevent heart failure》
3. Cell transplantation to prevent heart failure
Numerous clinical trials have demonstrated that cell transplantation was feasible and may have a marginal beneficial effect; however, ventricular function in clinical trials was not improved to the same extent as it was in the animal experiments. Among the variety of explanations for their limited benefit of cell transplantation in clinical trials, the most plausible is reduced cell potency and diminished endogenous repair mechanisms within elderly patients with multiple co-morbidities who have suffered a myocardial infarction [2,10]. Cell transplantation enhances the recovery of ventricular function by augmenting the endogenous repair mechanisms. However, elderly patients with extensive atherosclerosis frequently also have diabetes mellitus, obesity, and hypertension as well, which diminish the potency of their stem cells and their endogenous cardiac repair responses. Therefore, many clinical trials have attempted to enhance the capability of the transplanted cells to augment endogenous repair processes [11,12].
We selected highly potent stem cells with the hope that they would improve the recovery of ventricular function in elderly recipients with multiple co-morbidities in the implantation of autologous CD133+ stem cells in patients undergoing coronary artery bypass grafting (IMPACT CABG) trial [7]. Forty-one patients with ventricular dysfunction following a myocardial infarction were randomly assigned to have either highly potent CD133+ stem cells from their own bone marrow, or a similar-appearing placebo, injected into the infarct region of the heart during coronary artery bypass graft surgery. The injection of the cells or placebo is demonstrated in Fig. 1 [6]. All patients had cardiac magnetic resonance imaging both preoperatively and six months after surgery to quantify cardiac function and no differences were found between the groups. We found that it was feasible to harvest bone marrow cells, isolate the highly potent stem cell fraction, and inject those cells into the infarct region. However, cardiac function was not improved. A larger trial with more patients and a higher number of cells injected is planned in order to determine the potential value of this approach for stem cell therapy.
《Fig 1》

Fig 1. Injection of stem cells into the infarct region during coronary artery bypass surgery. Reproduced from Ref. [6] with permission of the American Association for Thoracic Surgery, © 2016.
In another trial we employed a different type of highly potent stem cell which was injected into the heart to sufficiently improve ventricular function in order to permit weaning from left ventricular assist support [13]. Nineteen sites in North America participated in this randomized trial. All patients were dependent on one of two approved left ventricular assist devices (Fig. 2) [13] to support their failing heart. These patients would require cardiac transplantation if their cardiac function did not improve sufficiently to be weaned from the device. Highly potent mesenchymal precursor stem cells (allogenic cells) were injected into the heart for this trial with the goal of improving ventricular function sufficiently to allow weaning from the assist device. One hundred and fifty-nine patients on a device were recruited for the trial and were randomized to receive either stem cells or a placebo. Each patient underwent a number of temporary attempts to wean from the assist device over the six-month period after the injections. No difference was found between the two groups in terms of their ability to be weaned from the device, and no increase in adverse events was found with the injection of the stem cells. Again, although this trial demonstrated that stem cell injection is feasible, the treatment was not efficacious. However, an evaluation of the effects of these stem cells was found to be substantially better in the subgroup with ischemic rather than non-ischemic cardiomyopathy. This secondary (but prespecified) subgroup analysis is sufficiently hypothesis generating to support further research. A higher dose of stem cells in patients with ischemic cardiomyopathy will be employed in future trials.
《Fig 2》

Fig 2. The two left ventricular assist devices employed in the trial of mesenchymal precursor cells to improve ventricular function sufficiently to be weaned from the assist device. (a) The study device—centrifugal-flow device (HeartWare; Medtronic, USA); (b) the control device—an axial-flow device (HeartMate II; Abbott Laboratories, USA). Reproduced from Ref. [13] with permission of the Massachusetts Medical Society, © 2017.
《4. Tissue engineering to restore ventricular function》
4. Tissue engineering to restore ventricular function
The wave of regenerative therapies developd to prevent progressive heart failure has stimulated a number of unique therapeutic approaches. Not only cells, but also tissue engineered constructs have been demonstrated to prevent infarct thinning and chamber dilatation to preserve ventricular function. Cardiac restraint devises were developed to prevent left ventricular dilatation after an infarction. For example, the Acorn (USA) device consists of a knitted polyester fabric that is positioned around the left ventricle to constrain the infarct area and prevent dilatation [14]. However, initial clinical trials were inconclusive, and this device is no longer available for clinical investigation.
Newer approaches involved injecting biomaterials into the infarcted region of the left ventricle to ‘‘buttress” the wall and prevent dilatation. The biological effects of the injected biomaterials have been extensively studied and are substantial [15]. This approach was evaluated in a variety of animal models and in clinical trials [16]. However, no single approach has been demonstrated to be uniformly effective, and no biomaterial has been released as a therapeutic agent. The initial clinical trials were inconclusive, although some studies suggested that the biomaterials altered matrix remodeling and favorably modified the healing of the infarct zone [17]. Aside from biomaterials, adding slowreleased cytokines (i.e., proteins to alter healing) was found to enhance new blood vessel formation (angiogenesis) and induce the recruitment of endogenous repair mechanisms [3,4]. Along with buttressing the ventricular wall, injected biomaterials were also found to enhance cell engraftment [4]. Therefore, the combination of biomaterials, cells, and cytokines may prove to be very effective.
One of our studies of biomaterials led to the development of temperature-sensitive and biodegradable aliphatic polyester hydrogel that is a liquid at room temperature but assumes consistency similar to that of gelatin at body temperature (37 °C) (Fig. 3) [3]. We also conjugated the hydrogel with a well-characterized cytokine which contributes to angiogenesis—namely, vascular endothelial growth factor (VEGF)—that contributes to angiogenesis after delayed release. These hydrogels, with and without conjugated VEGF, were injected into the infarct area 7 d after coronary ligation in a rat model. Both treatment groups exhibited improved ventricular function 42 d later, with the best functional restoration being achieved with the cytokine-enhanced hydrogel. This study indicates that biomaterials offer an attractive approach to improve function after myocardial infarction. However, the regulatory barriers are substantial as regulatory agencies require not only a delineation of all breakdown products of the materials, but also documentation of the biological effects each breakdown product.
《Fig 3》

Fig 3. An injectable scaffold consisting of a temperature-sensitive and aliphatic polyester hydrogel, conjugated with vascular endothelial growth factor, to enhance the recovery of ventricular function following a myocardial infarction. (a) Hydrogel is a liquid at room temperature and a gel at 37 °C; (b) electron micrograph illustrating that the hydrogel has a honeycomb-like structure with a pore size about 1 μm. Reproduced of Ref. [3] with permission of Elsevier Ltd., © 2011.
Although biomaterials induce physiological effects, which can be enriched with conjugated cytokines, tissue engineering can include both scaffolding and cellular components. Engraftment of cells in the myocardium is very limited, but the mixture of cells and hydrogels resulted in improved cellular engraftment [4].
《5. Surgical cardiac restoration and rejuvenation》
5. Surgical cardiac restoration and rejuvenation
One approach to surgically restore ventricular function after an extensive myocardial infarction was investigated in a large multi-center randomized trial [18]. The procedure involved excision of the mature scar after an infarction and reconstruction of the ventricle with a Dacron (USA) patch to reduce the ventricular chamber to a normal size and geometry. Unfortunately, the large clinical trial results was negative for efficacy. However, many surgeons claimed that only patients with excessively dilated ventricles benefitted from the procedure. The surgeons also suggested that some patients had been entered into the trial without the requisite cardiac dilatation, resulting in a lack of benefit. Some surgeons continue to perform this procedure in appropriate patients.
Our group in Toronto evaluated surgical ventricular restoration in a rat model using a biodegradable modified gelfoam (MGF) patch by itself, with only a hydrogel, or with a hydrogel seeded with stem cells and/or cytokines (Fig. 4) [19]. In the control group, the myocardium adjacent to the patch thinned and dilated, resulting in decreased cardiac function. Both cells and cytokines improved the recovery of ventricular function, with a combination of the biomaterial, cytokines, and cells resulting in the greatest recovery of ventricular function. These studies demonstrate that tissue engineering, which combines expertise in materials and biologicals, permits the creation of the best options for ventricular restoration.
《Fig 4》
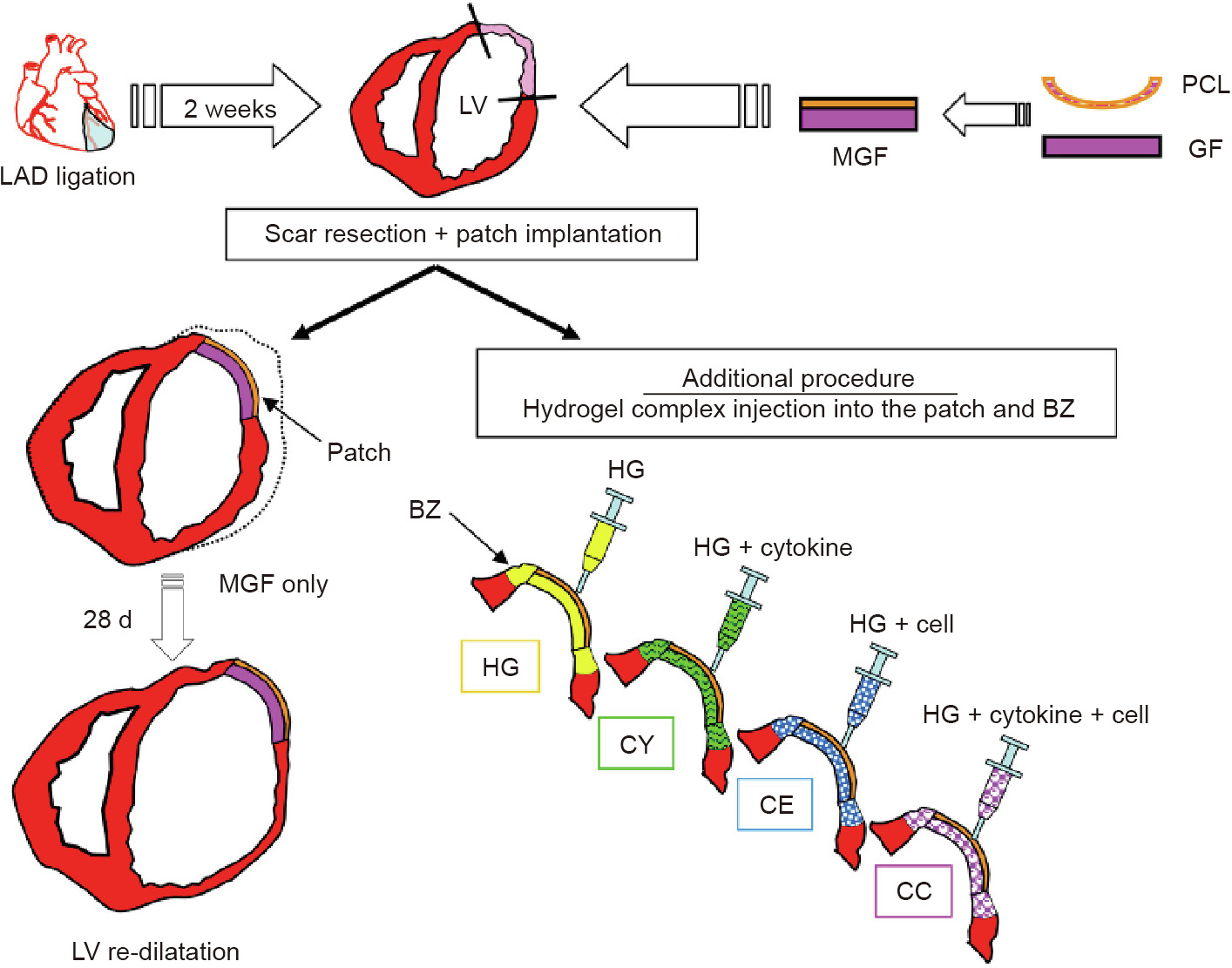
Fig 4. Surgical ventricular restoration with cells and cytokines to restore function to the damaged heart with an MGF patch, combined with hydrogel alone, or hydrogel with cells, and/or cytokines. LAD: left anterior descending; LV: left ventricular; PCL: poly( -caprolactone); GF: gelfoam; BZ: border zone; HG: hydrogel; CY: cytokines suspended in hydrogel; CE: bone marrow stem cells suspended in hydrogel; CC: cells and cytokines suspended in the hydrogel. Reproduced from Ref. [19] with permission of Elsevier Ltd., © 2010.
-caprolactone); GF: gelfoam; BZ: border zone; HG: hydrogel; CY: cytokines suspended in hydrogel; CE: bone marrow stem cells suspended in hydrogel; CC: cells and cytokines suspended in the hydrogel. Reproduced from Ref. [19] with permission of Elsevier Ltd., © 2010.
《6. The future of cardiac tissue engineering》
6. The future of cardiac tissue engineering
The restoration of ventricular function after a myocardial infarction remains an elusive goal for cardiac surgeons. Our studies have suggested that injection of a higher dose of autologous CD133+ cells might improve the recovery of ventricular function. Our studies also suggest that the injection of a higher dose of mesenchymal precursor cells may be beneficial for patients with ischemic cardiomyopathy who require a left ventricular assist device to maintain their circulation. A combination of biomaterials, cells, and cytokines added to the epicardium over the infarct region may prove to be beneficial during cardiac surgery; these clinical trials are under discussion for future implementation.
Tissue engineering combines engineering expertise about materials with knowledge of the biological processes required for restoring cardiac function after injury. However, the studies described above and the literature lack an understanding of cardiac electrical activation. Because the dense infarct scar is less conductive for electrical propagation than the normal myocardium, electrical activation is delayed and contractions become dyssynchronous contributing to diminished ventricular function following an infarction. In Toronto, Professor Ren-Ke Li has been studying a variety of biomaterials intended to enhance electrical propagation [20,21]. Integrating electrically-conducting components within the biomaterials enhances synchronous contraction and restores ventricular function. Thus, both the biological effects of the tissue engineering construct and its electrophysiological properties determine the beneficial effects that may be achieved.
《7. Summary》
7. Summary
Tissue engineering is the use of scaffolds together with biological elements to alter the healing processes. Understanding both engineering concepts and biological processes, permits the integration of engineering and biology to restore normal cardiac function. Combining engineering expertise with a medical understanding of physiology provides the best choices for our patients.













 京公网安备 11010502051620号
京公网安备 11010502051620号




