《1. Introduction》
1. Introduction
Over the past 100 years, the development of biodegradable materials has helped to advance innovation in tissue-engineering technology by making such materials more feasible, resulting in technological breakthroughs and new clinical applications [1,2]. Classical tissue-engineering theory involves three elements: biological materials, seed cells, and factors. Research progress suggests that biodegradable materials with low immunogenicity, high biodegradability, good biocompatibility, and favorable regeneration microenvironments are of great significance [3].
Nerve injury is a common clinical problem across the world. Peripheral nerve defects and spinal cord injuries (SCIs) result in a high rate of disability, which worsens patient quality of life and burdens families and society. Therefore, the development of new biodegradable materials that can help to more effectively repair peripheral nerve defects and SCI—as well as mediate functional rehabilitation in tissue-engineered nerves—has been a major issue in the fields of materials science, neuroscience, tissue engineering, and regenerative medicine.
《2. Biodegradable materials》
2. Biodegradable materials
In the field of nerve transplants, the term ‘‘biodegradable materials” refers to natural or synthetic biomedical materials that can be degraded, absorbed, or excreted by the body upon coming into contact with internal fluids, acids, or enzymes—such as when entering metabolic and biochemical reactions. Ultimately, the implanted materials are completely replaced by new tissues. A biodegradable scaffold must satisfy several important criteria to provide a suitable environment for axon regeneration. Recently, numerous classes of biodegradable materials have served as scaffolds for neural tissue engineering, including polyesters (e.g., poly(glycolic acid) (PGA), poly(lactic acid) (PLA), poly(lactic acid–glycolic acid) (PLGA), poly(ε-caprolactone) (PCL), and polyurethanes (PUs)). Additional materials include natural fibrin, collagen, keratin, alginate, chitin and chitosan, and silk fibroin, as well as extracellular matrix (ECM) and extracellular vesicles (EVs) (Fig. 1) [4,5].
《Fig. 1》
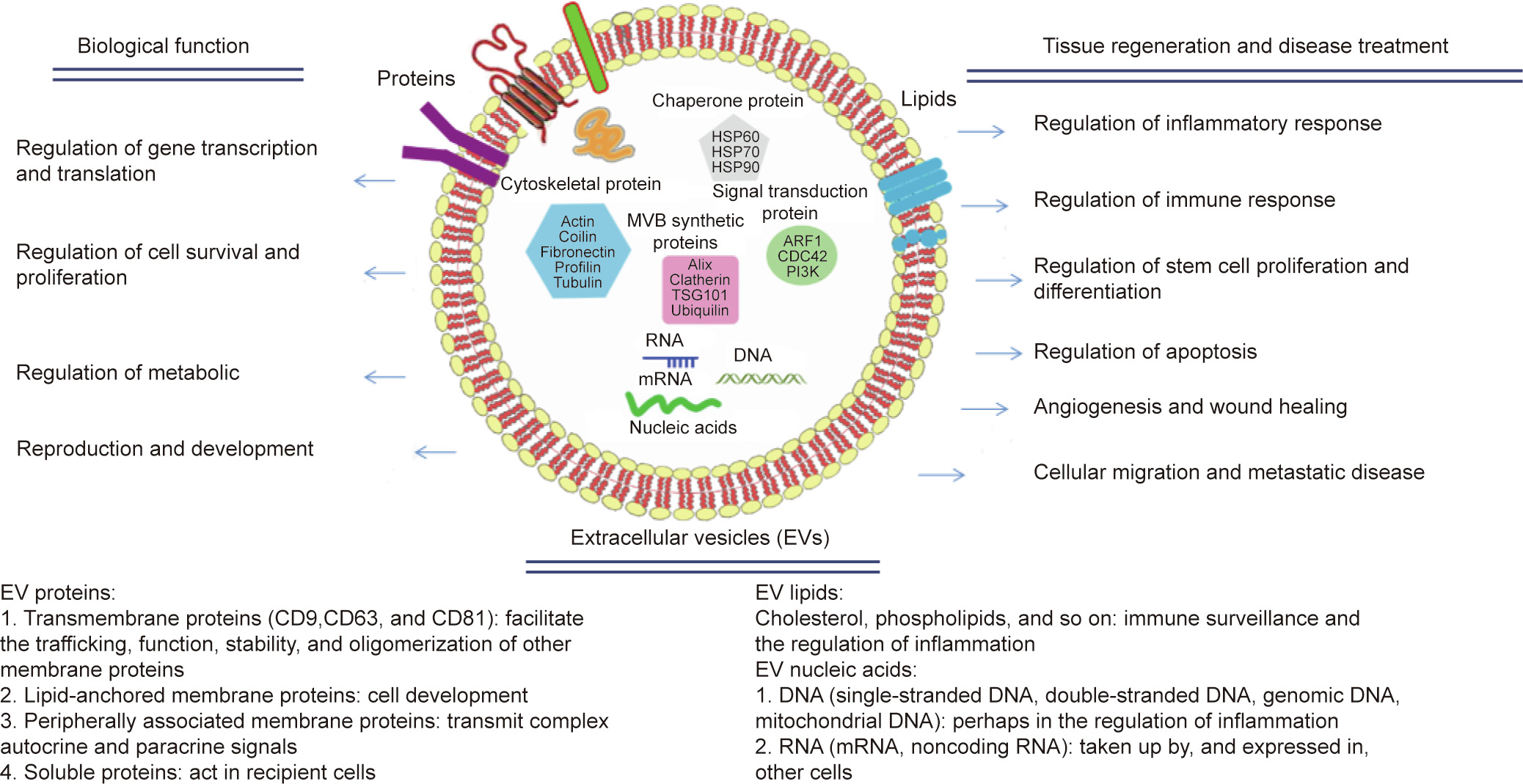
Fig. 1. Extracellular vesicles (EVs). HSP: heat shock protein; MVB: multivesicular body; TSG: tumor-susceptibility gene; ARF: adenosine diphosphate (ADP)-ribosylation factor; CDC: cell division cycle; PI3K: phosphatidylin-ositol-3-kinase; mRNA: messenger ribonucleic acid (RNA); CD: cluster of differentiation. Part data reprinted and adapted from Refs. [4,5].
The ECM plays an extremely significant role in constructing and maintaining an active microenvironment for tissue regeneration. As a feasible alternative, ECM scaffolds are used to provide therapy at the cellular and molecular level in tissue engineering [6]. Structurally, the ECM creates unique forms and topographies on a nano-, micro-, and macro-scale, and provides a mechanical framework that permits cell–cell interactions for healthy tissue formation and maintenance. EVs are natural nano-sized to microsized membrane vesicles that are encapsulated by phospholipid bilayers and actively released by cells. According to their function, size, and structure, EVs are divided into three categories: apoptotic bodies, microvesicles, and exosomes [7]. Recipient cells respond to EV-loaded molecules with changes in gene expression that can affect cell function when EVs are internalized [8]. EVs can act as natural therapeutic ingredients for treating many common and intractable diseases [5], and EVs modified in vitro have strong anti-inflammatory and axon-regeneration ability [9].
In tissue engineering for cartilage regeneration, the integration of nanomaterials can lead to the development of scaffolds that better simulate the ECM environment of cartilage, thereby enhancing the interaction between scaffold and cells, and improving the function of engineering organization construction. This technique can be used not only for the treatment of focal defects, but also to address the extensive degenerative changes caused by osteoarthritis in joints [10]. Hyaluronic acid (HA) and its derivatives have been applied in tissue engineering due to their unique chemical and structural properties [11], and have attracted a great deal of attention in the field of regenerative medicine.
In recent years, the development of various nanoscale strategies has provided new approaches for the treatment of SCI by crossing the blood–spinal cord barrier and transmitting therapeutic agents. Song et al. [12] have discussed different fabrication methods for nanomaterials and reviewed the recent research progress of nanomaterials for regulating inflammatory signals, targeting inhibitors, and promoting axonal regeneration after SCI. In order to overcome cytotoxicity and the side-effects of the system, and to provide more effective therapeutic drugs, researchers have developed various nanocarriers that allow the local, slow, and sustained delivery of therapeutic drugs to the injured site. Numerous studies have shown that drug delivery through nanocarriers at the cellular and behavioral levels in pre-clinical models can achieve similar therapeutic effects to traditional methods at a low dose or the same dose.
Apart from drug delivery, providing sub-cellular nanomorphology is the key to nerve-repair strategy. Nanotechnology provides an attractive strategy for extending stability and penetrating the blood–brain barrier (BBB), while selectively transmitting nutrients to pathologically related cells. Nanoscaffolds are produced to simulate the structural and functional characteristics of the ECM as much as possible. They not only provide mechanical support, but also play a very important role in the regulation of cell adhesion, proliferation, differentiation, and migration. Due to the high complexity of nerve regeneration, the strategy of integrating many therapeutic targets in cells and the ECM constitutes a more holistic approach, which may produce better therapeutic effects.
Nanotechnology approaches can be combined and designed to address the pathology of multiple diseases within a nanosystem [13]. These data in Ref. [13] suggest that nanoparticles provide a platform to limit acute inflammation and tissue destruction with a good risk–benefit ratio, resulting in a regeneration microenvironment that supports regeneration and functional recovery. Inflammatory reactions, which typically occur in response to SCI, cause extensive tissue damage and damage function. Polymer nanoparticles are formed in the absence of active drug components, which can produce an off-target effect. This internalization redirects some immune cells to the spleen, with modest numbers at the SCI. After intravenous injection, the infiltration of immune cells decreases, which is related to the decrease of tissue degeneration. In addition, SCI has developed into a complex injury response and a permissive microenvironment characterized by the phenotype of regenerative immune cells, the expression of regenerative related genes, the increase of axons and myelin, and the significant improvement of functional recovery. These nanoparticles may be applied to many inflammatory diseases.
With low toxicity and good blood/tissue compatibility, edaravone-carried and angiopep-2 (ANG)/poly(ethylene glycol) (PEG)-conjugated ceria nanoparticles (E-A/P-CeO2) can be used to effectively treat stroke through excellent brain-uptake enhancement and effective BBB protection. It can also reduce harmful side-effects and sequelae. Recent studies demonstrated a new way to improve drug delivery to ischemic brain tissue and established a new glyburide preparation that could potentially be converted to clinical application in order to improve the management of human stroke patients. A new method of encapsulating nanoparticles with an engineered cell membrane was proposed to target brain drugs [14]. Recent findings suggest that oriented and rigid electrospun nanofiber scaffolds exhibit considerable potential for nerve injury repair.
Nanomaterials can be used to stimulate nerve growth. The combination of hydrogels with nanomaterials such as carbon nanotubes (CNTs) yields the required performance, and CNT-based scaffolds show significant ability to guide neural network connections. A clinical-related adult rat spinal cord contusion model was used to investigate the mechanical support of nanohybrid composite (NHC) and its effect on neurogenesis by evaluating the presence of macrophages, blood vessels, axons, and nerve-like cells. The researchers reported that their composites provided mechanical support for the SCI segment as well as a microenvironment that was conducive to endogenous cell infiltration, which is important for the repair and generation of nerve tissue [15]. Recent studies have analyzed the potential application of biomimetic multi-channel neuro-guided conduits based on shape-memory nanofibers in peripheral nerve repair. Based on the characteristics of the shapememory poly(L-lactide-co-trimethylene carbonate) (PLATMC) polymer, a self-forming multi-channel nerve-guided catheter was successfully prepared. The tube-forming process is simple and fast, and the inner diameter is easy to control, which meets the actual size requirements of the repaired nerve. In recent years, nanotechnology has played a huge role in peripheral nerve repair (PNR). By developing advanced nanosystems (e.g., oriented nanofibers or CNTs) to solve the short-gap and long-gap problems at the same time, these nanosystems can guide and stimulate the correct regrowth of axons. Progress in the application of nanomedicine in PNR is expected to have a significant impact on the rehabilitation and quality of life of patients, because new treatment strategies are evolving and reflect the interdisciplinary and comprehensive treatment strategies that integrate nanotechnology with tissue engineering.
《3. Biodegradable materials in peripheral nerve tissue engineering》
3. Biodegradable materials in peripheral nerve tissue engineering
To assist in the repair of nerve injuries, biomaterials are usually modified and improved. Based on an assessment of the devices reported in the literature, these devices can be classified into three classes: isotropic hydrogel fillers, which provide interluminal support for nerve regeneration; fibrous interluminal fillers, which offer intraluminal topographical guidance for neurites; and patterned interluminal scaffolds, which provide nerve growth with threedimensional (3D) structural support.
Silk fibroin (SF), which is derived from natural silk, has become a significant biomaterial for tissue-engineering applications in recent years. SF-based nerve grafts have been used for peripheral nerve regeneration [16]. The incorporation of EVs from skinderived precursor Schwann cells into nerve grafts is a promising paradigm for the repair of peripheral nerve injury [17].
《4. The use of biodegradable materials in the repair of spinal cord injury》
4. The use of biodegradable materials in the repair of spinal cord injury
For biological applications, it is important to consider the properties of the scaffold. The ideal scaffold for the repair of SCIs should have the following characteristics:
(1) Biocompatibility. Neither the polymer itself nor its degradation products should cause inflammation or be toxic.
(2) Biodegradability. The degradation rate should match the rate of nerve growth, and the degradation products should eventually be removed.
(3) Permeability or porosity. Appropriate porosity provides enough space for the ECM and promotes cell adhesion. This allows cells to be distributed throughout the scaffold, which facilitates regeneration.
(4) Cell adhesion and growth. The surface permits cell adhesion and promotes cell growth.
(5) Biomechanical properties. The scaffold should have a 3D structure with considerable mechanical strength.
With the continuous release of nerve growth factor (NGF), which is achieved by means of a microsol core–shell structure, immunological fiber scaffolds have been shown to result in a remarkable shift in immune cell subtype, which downregulated the acute inflammation response, affected scar tissue formation, promoted angiogenesis and neural differentiation at the injury site, and promoted functional recovery in a spinal cord hemi-section model on a Sprague–Dawley (SD) rat [18]. The biodegradable hybrid inorganic (BHI) scaffold significantly improved the survival rate of transplanted human-induced pluripotent stem-cell-derived neural stem cells (hiPSC-NSCs), promoted their differentiation into neurons, and inhibited the formation of scar tissue [19].
Recent studies demonstrated that neurotrophin-3 (NT-3)- loaded chitosan biodegradable material enabled the prolonged slow release of NT-3 for 14 weeks under physiological conditions—a finding that illustrates both NT-3-chitosan-enabled robust neural regeneration and motor and sensory functional restoration. Given the similar genetics and physiology between monkeys and humans, it is probable that technologies that have successfully been applied for SCI repair in monkeys will be translatable to human SCI repair [20].
《5. Conclusions and prospects》
5. Conclusions and prospects
The field of biomaterial science has promoted the development of tissue engineering and regenerative medicine. To make tissue-engineering research more applicable in clinical settings, it is proposed that the following key elements be considered when searching for or engineering new materials for use in transplants: ① biodegradable materials, ② stem cells or supporting cells, ③ growth factors or cytokines, ④ cell matrix, and ⑤ a regenerative microenvironment.
The prospects for the use of biodegradable materials in a new generation of biomimetic devices are good. According to recent studies, in addition to the chemical composition of biodegradable materials, research should focus on growth factor bionics, micro– nano architecture bionics, and the biomimetic regeneration microenvironment of biomimetic materials (Fig. 2). The interactions between biomaterials and host cells and tissues, as well as the complex bidirectional regulations, need to be explored and understood [21].
《Fig. 2》

Fig. 2. Construction of biomimetic tissue-engineering nerves. NGF: nerve growth factor; BDNF: brain derived neurotrophic factor; GDNF: glial derived neurotrophic factor; CNTF: ciliary neurotrophic factor.
Good progress has been made in the use of tissue engineering for the repair of peripheral nerve defects (Table 1). However, further efforts are needed toward the repair and functional reconstruction of long-distance nerve-trunk defects.
《Table 1》
Table 1 Institutions with at least 11 published articles related to biodegradable materials and neural tissue engineering.

Institutions are ranked by number of publications. From 2001 to 2020, there were 1300 publications related to biodegradable materials and neural tissue engineering.
Methods for the repair and regeneration of SCI can be divided into biological and engineering subtypes. Biological methods use biodegradable materials, soluble bioactive molecules, the cell matrix, and cell transplantation. Engineering techniques include spinal epidural electrical stimulation, deep brain stimulation, and brain–computer interface systems.
The field of global SCI repair has developed rapidly. However, from a clinical perspective, the number of clinical trials related to this topic is small. At present, most clinical trials for SCI repair and regeneration have not yet reached phase III, and the objective clinical efficacy of the methods involved remains to be verified.
New biodegradable materials, tissue-engineering methods, and nerve-regeneration techniques are continuously emerging with progress in the fields of materials science, neuroscience, biomedical engineering, tissue engineering, and regenerative medicine. Based on the key technology of bionic tissue-engineering construction, final realization of the construction of bionic tissue and bionic organs and their successful transformation into clinical application could bring significant benefits for human health. The repair of long-distance nerve defects and the functional reconstruction of SCI remain the goals of human medical research in this field. Further strategies should be addressed in order for patients to regain better functionality. These strategies must be promoted jointly by multidisciplinary experts, scholars, and teams, along with multi-field laboratory technology integration, continuous investment in research funds, the administration of standards guides, and collaborative innovation based on globalization [22].
《Acknowledgments》
Acknowledgments
I am grateful for helpful suggestions from Zhaolian Ouyang at the Institute of Medical Information/Medical Library, Chinese Academy of Medical Sciences and Peking Union Medical College and contribution to the writing from Lai Xu at Nantong University.
This work was supported by the National Natural Science Foundation of China (31730031 and L1924064) and the Natural Science Foundation of Jiangsu (BK20202013).














 京公网安备 11010502051620号
京公网安备 11010502051620号




