《1. Introduction》
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first caught the world’s attention in an atypical pneumonia [1]. Being highly transmissible, the novel coronavirus disease caused by this virus—namely, coronavirus disease 2019 (COVID-19)—has become an overwhelming threat to global public health [2,3]. The global morbidity and mortality of COVID-19 continue to rise. As of 20 July 2021, COVID-19 has been confirmed in over 190 million people worldwide and has caused over 4 million deaths [4]. In response to the global COVID-19 pandemic, researchers in medicine, public health, pharmaceutics, and many other related fields have made great efforts to develop effective therapeutic and disease-controlling methods. Accumulating evidence suggests that a subgroup of COVID-19 patients may experience cytokine storm syndrome, the occurrence of which is linked to an increase in mortality [5]. Inflammation, which is the primary host defense in response to viral infection, is marked by the activation and mobilization of a variety of inflammatory cells, including macrophages, neutrophils, and lymphocytes. Overactivation of inflammatory cells may result in the uncontrolled release of cytokines and detrimental systemic immune response, in what is known as ‘‘cytokine storm.” Therefore, it is important to identify and suppress hyperinflammation in this subgroup of COVID-19 patients at an early stage.
Xuanfeibaidu Formula (XFBD) is a first-line traditional Chinese medicine (TCM) formula used for COVID-19 patients with symptoms of ‘‘Shi Du Yu Fei” (the lung is stagnated by noxious dampness), designed by the Chinese Academy of Engineering (CAE) academician Boli Zhang and Professor Qingquan Liu [6]. A pilot randomized clinical trial suggested that XFBD, combined with conventional antiviral medicine, significantly accelerated the resolution of clinical symptoms in COVID-19 patients, compared with the symptoms in patients treated with antiviral drugs alone [7]. XFBD is composed of 13 medicinal herbs and natural materials, and is intended to possess multi-target effects, according to TCM theory [6]. It is notable that an in silico pharmacological analysis suggested that nearly 10% of the putative XFBD targets were enriched in the disease pathways of viral infection and lung injury, with immunity and inflammation being among the major biological pathways regulated by the targets [6]. However, the pharmacological mechanism of XFBD still needs to be tested.
The major difficulty encountered in XFBD pharmacological studies, as in studies of many other TCMs, is the complexity of its chemical composition and biological activities. To address this challenge, we proposed the necessity of multimodal identification of TCM active constituents, which emphasizes information acquisition and fusion from multiple study models at different scales. In the present study, we integrated information from network pharmacology results, based on high-resolution mass spectrometry (MS) experiments with a transcriptomics analysis of a mice inflammation model in order to identify potential targeting pathways of XFBD (Figs. S1 and S2 in Appendix A). Next, with a focus on the predictive roles of XFBD in macrophage-mediated hyperinflammation, we further examined the pharmacological effects of XFBD and its active components in both in vitro and in vivo systems. Taken together, our findings identify the regulatory effect of XFBD and its components in the pathological process of inflammationstimulated macrophage migration and cytokine secretion, which partially explains the pharmacological mechanism of XFBD in manipulating immune hyperactivation.
《2. Methods》
2. Methods
《2.1. Materials and reagents》
2.1. Materials and reagents
XFBD granules and individual herb extracts were provided by Tianjin Modern Innovative TCM Technology Co., Ltd. (China). Chlorogenic acid, ferulic acid, 3,4-dicaffeoylquinic acid, 3,5- dicaffeoylquinic acid, naringenin, kaempferol, atractylenolide I, emodin, pogostone, neochlorogenic acid, amygdalin, isoschaftoside, verbenalin, hastatoside, rosmarinic acid, isoacteoside, isoliquiritin, apigenin, catechin, quercetin-3-O-β-D-glucose-7-O-β-D-gentiobioside, cryptochlorogenic acid, acteoside, naringin, hesperidin, isoliquiritin apioside, glycyrrhizic acid, and artemisinin were purchased from Shanghai Winherb Medical Technology Co., Ltd. (China). Adenosine, atractylenolide III, and ononin were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (China). Luteolin was purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. (China). Schaftoside and 4,5-di-O-caffeoylquinic acid were purchased from Chengdu MUST Bio-Technology Co., Ltd. (China). Ephedrine hydrochloridum was obtained from the National Institutes for Food and Drug Control (China). Resveratrol was acquired from Sigma–Aldrich (USA). The purities of all compounds were greater than 98%. All compounds were dissolved in dimethyl sulfoxide (DMSO) to form stock solutions with a concentration of 100 mmol·L–1.
High-glucose Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), trypsin–ethylenediaminetetraacetic acid (EDTA), and antibiotics (100 units·mL–1 of penicillin G and 100 g·mL–1 of streptomycin) were obtained from Gibco BRL (USA). A mouse peripheral blood mononuclear cell (PBMC) isolation kit was purchased from Haoyang Biological Manufacturing Co., Ltd. (China). Poly(I:C) and Pam3CSK4 were purchased from Invivogen (USA). An interleukin (IL)-6 enzyme-linked immunosorbent assay (ELISA) kit was obtained from Boster Biological Technology Co., Ltd. (China). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mouse monoclonal antibody, cell lysis buffer, and phenylmethylsulfonyl fluoride (PMSF) for western blotting were purchased from Beyotime Biotechnology (China).
《2.2. Preparation of XFBD and herbs test solution》
2.2. Preparation of XFBD and herbs test solution
A sample of XFBD granules with a mass of 119 g contains the following components: 3 g of Ephedrae Herba (Ephedrae H.), 7.5 g of Armeniacae Semen (Armeniacae S.), 15 g of Gypsum Fibrosum (Gypsum F.), 15 g of Coicis Semen (Coicis S.), 5 g of Atractylodis Rhizoma (Atractylodis R.), 7.5 g of Pogostemonis Herba (Pogostemonis H.), 6 g of Artemisiae annuae Herba (Artemisiae H.), 10 g of Polygoni cuspidati Rhizoma (Polygoni R.), 15 g of Verbenae Herba (Verbenae H.), 15 g of Phragmitis Rhizoma (Phragmitis R.), 7.5 g of Lepidii/ Descurainiae Semen (Lepidii/Descurainiae S.), 7.5 g of Citri grandis Exocarpium Rubrum (Citri grandis E.), and 5 g of Glycyrrhizae Radix (Glycyrrhizae R.). The XFBD granules were dissolved in water to form a stock solution with a concentration of 200 mg·mL–1 .
For the individual herb extracts, 100 g of each herb material (or 50 g for Ephedrae H.) was added to 1.5 L water and extracted by reflux extraction for 1 h, separately. All the extracts were concentrated by reducing the pressure and freeze-drying. The extracts were dissolved in water to form stock solutions with a concentration of 1 mg·mL–1 .
《2.3. Liquid chromatography quadrupole time-of-flight mass spectrometry (LC-Q-TOF-MS) analysis of XFBD extract》
2.3. Liquid chromatography quadrupole time-of-flight mass spectrometry (LC-Q-TOF-MS) analysis of XFBD extract
An ACQUITY ultra-performance liquid chromatography (UPLC) system (Waters, USA) coupled with a triple time-of-flight (TOF) 5600+ MS (AB SCIEX, USA) and equipped with an electrospray ionization (ESI) source was employed for chemical identification. Samples were separated on a Waters ACQUITY UPLC HSS T3 (150 mm × 2.1 mm; internal diameter (i.d.), 1.8 μm) at a flow rate of 0.3 mL·min–1 and a column temperature of 50 °C. The mobile phases were as follows: 0.1% formic acid–water as an aqueous mobile phase (A) and 0.1% formic acid–acetonitrile as an organic mobile phase (B). The linear gradient elution was optimized as follows: 0–2 min, 0% B; 2–25 min, 0%–30% B; 25–35 min, 30%–95% B; 35–37 min, 95% B. The injection volume was 2 μL and the detection wave was 254 nm. An MS analysis was performed in both positive and negative modes under the following parameters: scan range, mass-to-charge ratio (m/z) scan ranges 100–2000 Da (negative), 90–1500 Da (positive); ion source GS1, 50 psi (1 psi = 6.89 kPa); ion source GS2, 50 psi; curation gas (CUR), 35 psi; temperature, 600 °C for positive-ion ESI (ESI+) and 550 °C for negative-ion ESI (ESI–) ionspray (IS) voltage, –4.5 kV for ESI– and 5.5 kV for ESI +; declustering potential (DP), 100 V; and collision energy (CE), 10 V.
《2.4. Molecular networking construction of XFBD》
2.4. Molecular networking construction of XFBD
The tandemMS (MS2 ) data files were converted from the .wiff (AB SCIEX) standard data format to the .mzXML format using MSConvert software, within the ProteoWizard package. All .mzXML data were then processed using MZmine 2 v.40.1 [8]. MS detection was performed by setting the noise level at 5.0 × 104 . The automated data analysis pipeline (ADAP) chromatogram builder was used, with a set of a minimum scan group size of 5, a group intensity threshold of 5.0 × 104 , a minimum highest intensity of 5.0 × 104 , and an m/z tolerance of 0.001 Da or 10 parts per million (ppm). The ADAP wavelets deconvolution algorithm [9] was used: signal to noise (S/N) threshold = 10, minimum feature height = 5.0 × 104 counts, coeffcient/area threshold = 100, peak duration range 0–1 min, retention time (RT) wavelet range 0–0.2 min. MS2 scans were paired using an m/z tolerance range of 0.02 Da and an RT tolerance range of 0.1 min. An isotopic peaks grouper algorithm was used, and peak alignment was performed using the join aligner module. The peak list was gap-filled with the peak finder module and then filtered using the peak list row filter. Eventually, the .mgf data file and its corresponding .csv metadata file, including peak height and area integration, were exported.
Corresponding molecular networking was created according to the online workflow at Global Natural Products Social Molecular Networking (GNPS)↑ [10] with a parent mass tolerance of 0.02 Da, an MS2 fragment ion tolerance of 0.02 Da, and a minimum cluster size of 1. MScluster was run and the filter precursor window tools were turned off. The network that was created had a cosine score of above 0.7 and more than four matched peaks. The spectra in the network were then searched against the GNPS spectral libraries. The molecular networking data were visualized using Cytoscape (ver.3.7.2).
↑ http://gnps.ucsd.edu.
《2.5. Compounds-targets network of XFBD》
2.5. Compounds-targets network of XFBD
The targets of the main compounds of XFBD were collected from the Encyclopedia of Traditional Chinese Medicine (ETCM) , Traditional Chinese Medicine Systems Pharmacology (TCMSP)↑↑, and TargetNet
, Traditional Chinese Medicine Systems Pharmacology (TCMSP)↑↑, and TargetNet databases (until 31 March 2020). The criteria for the chosen targets were the following: a value above 0.8 in ETCM; an area under the receiver operating characteristic curve (AUC) ≥ 0.7, a probability (Prob) > 0.9 in TargetNet; or an oral bioavailability (OB) ≥ 30% or a drug likeness (DL) ≥ 0.18 in TCMSP. The results were then normalized as follows: Targets’ names from ETCM were standard gene names; targets’ names from TargetNet were converted to standard gene names using the Uniprot website; and targets’ names from TCMSP were converted using bioDBnet. All the database retrieval results were summarized to build a compounds-targets corresponding database.
databases (until 31 March 2020). The criteria for the chosen targets were the following: a value above 0.8 in ETCM; an area under the receiver operating characteristic curve (AUC) ≥ 0.7, a probability (Prob) > 0.9 in TargetNet; or an oral bioavailability (OB) ≥ 30% or a drug likeness (DL) ≥ 0.18 in TCMSP. The results were then normalized as follows: Targets’ names from ETCM were standard gene names; targets’ names from TargetNet were converted to standard gene names using the Uniprot website; and targets’ names from TCMSP were converted using bioDBnet. All the database retrieval results were summarized to build a compounds-targets corresponding database.
 http://www.tcmip.cn/ETCM.
http://www.tcmip.cn/ETCM.
↑↑ https://tcmspw.com/.
 http://targetnet.scbdd.com/.
http://targetnet.scbdd.com/.
Next, the disease genes of COVID-19 were collected from GeneCards↑↑↑ , the Comparative Toxicogenomics Database (CTD) , PubMed Gene↑↑↑↑, and Online Mendelian Inheritance in Man (OMIM)
, PubMed Gene↑↑↑↑, and Online Mendelian Inheritance in Man (OMIM) .
.
↑↑↑ https://www.genecards.org/.
 http://ctdbase.org/.
http://ctdbase.org/.
↑↑↑↑ https://www.ncbi.nlm.nih.gov/gene/.
 https://www.omim.org/.
https://www.omim.org/.
A total of 5556 co-expressed genes of ACE2, which were confirmed to act as receptors of SARS-CoV-2, were acquired from GeneMANIA↑↑↑↑↑ and normalized to transform them into the standard gene names of Homo sapiens. All the genes were uploaded to the STRING database to obtain the correlation of each gene and were visualized by Cytoscape (ver.3.7.2). Biological functions of the critical targets were analyzed by the STRING database and the Database for Annotation, Visualization, and Integrated Discovery (DAVID)↑↑↑↑↑↑.
database to obtain the correlation of each gene and were visualized by Cytoscape (ver.3.7.2). Biological functions of the critical targets were analyzed by the STRING database and the Database for Annotation, Visualization, and Integrated Discovery (DAVID)↑↑↑↑↑↑.
↑↑↑↑↑ http://genemania.org/.
 https://string-db.org.
https://string-db.org.
↑↑↑↑↑↑ https://david.ncifcrf.gov/.
《2.6. Lipopolysaccharide (LPS)-induced C57BL/6 mice model and XFBD treatment》
2.6. Lipopolysaccharide (LPS)-induced C57BL/6 mice model and XFBD treatment
C57BL/6 mice that were 6–8 weeks old were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (China) and were randomly assigned into four groups—control, LPS, low-dose XFBD, and high-dose XFBD—with 10–15 mice per group. The modeling strategy of LPS-induced acute inflammation in mice has been reported previously [11–13]. In brief, the mice in the control and LPS groups were orally treated with saline for 3 days, and the mice in the LPS group were intraperitoneally injected with LPS (20 mg·kg–1 ) on the fourth day for 4 h. In clinical practice, the daily dosage of XFBD is about 8.6 g·kg–1 body weight [7]. In our study, the mice in the XFBD-treated groups were orally treated with XFBD (low: 2.16 g·kg–1 ; high: 4.32 g·kg–1 ) for 3 days, and then intraperitoneally injected with LPS (20 mg·kg–1 ) on the fourth day together with XFBD for 4 h. The mice were anesthetized and sacrificed, and the blood and lungs were collected. The blood samples of at least six mice were centrifuged to obtain serum. The concentrations of IL-6, tumor necrosis factor (TNF)-α, and IL-1β in the serum were measured by means of an ELISA kit according to the manufacturer’s instructions. The mononuclear cells in the blood samples from another six mice were collected by means of a PBMC isolation kit, and every two samples in the same group were combined into one for transcriptome sequencing. Lung tissue samples were fixed and stained by hematoxylin and eosin (H&E). All animal experiments were performed in accordance with the Guide for the care and use of laboratory animals (1996) published by the US National Institutes of Health (NIH) and were approved by the Institutional Animal Care and Use Committee of Zhejiang University.
《2.7. In vivo constituents and metabolites analysis of XFBD》
2.7. In vivo constituents and metabolites analysis of XFBD
The serum and lung samples obtained from the LPS-induced and XFBD-treated (2.16 g·kg–1 ) mice were processed for in vivo constituents and metabolites analysis. Serum samples (60 μL) were added to four times their volume of methanol for protein precipitation. The mixture was centrifuged at 12 000 revolutions per minute (rpm) for 10 min, and then the supernatant was evaporated to dryness. The residue was dissolved with 150 μL of 80% methanol for LC-Q-TOF-MS analysis using the same method that was applied for the extracts. Lung samples were homogenized in five times their volume of 80% methanol, and the homogenates were centrifuged at 12 000 rpm for 15 min. The supernatant was then used for LC-Q-TOF-MS analysis. The MS data were compared with prior MS analysis of XFBD to identify the constituents.
For quantitative analysis, 100 μL serum samples were added with four times their volume of methanol for protein precipitation. The whole lung tissue samples were homogenized in 1 mL of 80% methanol. After being centrifuged, the supernatants for the serum and lung samples were evaporated to dryness; finally, the residues were all dissolved with 100 μL of 80% methanol for LC-Q-TOF-MS analysis.
《2.8. Transcriptomic analysis of mononuclear cells from LPS-induced and XFBD-treated mice》
2.8. Transcriptomic analysis of mononuclear cells from LPS-induced and XFBD-treated mice
A differential expression analysis of mononuclear cells from LPS-treated and XFBD-treated group was performed using the DESeq2 R package (version 1.16.1). DESeq2 provides statistical routines for determining differential expression in digital gene expression data using a model based on the negative binomial distribution. The resulting P values were adjusted using Benjamini and Hochberg’s approach [14] for controlling the false discovery rat. Genes with an adjusted P < 0.05 found by DESeq2 were assigned as differentially expressed. A protein–protein interaction (PPI) analysis of differentially expressed genes was performed on the STRING database. Pathway enrichment was performed using DAVID Bioinformatics Resources 6.8↑ .
↑ https://david.ncifcrf.gov/tools.jsp.
《2.9. Macrophage activation model for anti-inflammation activity evaluation》
2.9. Macrophage activation model for anti-inflammation activity evaluation
RAW 264.7 cell lines were purchased from the Shanghai Cell Bank of Chinese Academy of Sciences (China) and cultured in high-glucose DMEM with 10% FBS and antibiotics (100 units·mL–1 of penicillin and 100 μg·mL–1 of streptomycin). The cells were seeded in 12-well plates at a density of 100 000 cells. After 1 day, the cells were co-incubated with Poly(I:C) (10 μg·mL–1 ) +- Pam3CSK4 (100 ng·mL–1 ) (P2P) and drug tested for 24 h. The concentrations were set as follows: Atractylodis R., Citri grandis E., and Phragmitis R., 800, 400, and 200 μg·mL–1 , respectively; Armeniacae S., Polygoni R., and Glycyrrhizae R., 400, 200, and 100 μg·mL–1 , respectively; Lepidii/Descurainiae S., 200, 100, and 50 μg·mL–1 ; Coicis S., 50, 25, and 12.5 μg·mL–1 ; Artemisiae H., 4, 2, and 1 μg·mL–1 ; Verbenae H., 2, 1, and 0.5 μg·mL–1 ; Pogostemonis H., 1, 0.5, and 0.25 μg·mL–1 ; and Ephedrae H., 0.125, 0.0625, and 0.0313 μg·mL–1 . These concentrations of herbal extracts and compounds were determined in combination, according to their toxicity to the cells and our previous experiences [15]. As the positive control, 1.2 μmol·L–1 of dexamethasone (DEX) treatment was used for 24 h. After that, the cell culture medium was collected for IL-6 concentration measurement using an ELISA kit, according to the manufacturer’s instructions.
《2.10. Western blotting》
2.10. Western blotting
RAW 264.7 cells (3 × 105 cells per well) pretreated with compounds for 24 h were treated with P2P for 1 h. Cell lysates were generated by means of a lysis buffer containing protease and phosphatase inhibitors. The lysates were prepared with loading buffer and separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% bovine serum albumin (BSA) and incubated with specific primary antibodies overnight at 4 °C. After being washed three times with tris buffered saline with Tween 20 (TBST), the membranes were incubated with the respective second antibodies at room temperature for 1 h, and subsequently washed. The signal of membranes were detected with chemiluminescence. Finally, an analysis of band density was carried out using Image Lab v5.2 software (BioRad, USA).
《2.11. Zebrafish tail amputation and pharmacological treatment》
2.11. Zebrafish tail amputation and pharmacological treatment
Tg(mpeg:eGFP) [16] and Tg(Lyz:dsRED2) [17] transgenic zebrafish were obtained from the Laboratory Animal Center of Zhejiang University. Zebrafish were maintained following standard protocols [18]. E3 medium (0.29 g·L–1 of NaCl, 0.013 g·L–1 of KCl, 0.048 g·L–1 of CaCl2·2H2O, and 0.082 g·L–1 of MgCl2·6H2O, at pH 7.2) was used as the embryo medium. Embryos were obtained through natural spawning. All zebrafish experiments were conducted according to the guidelines of the Animal Ethics Committee of the Laboratory Animal Center, Zhejiang University.
For drug pretreatment, 5 days post-fertilization (dpf) of zebrafish embryos were incubated in embryo medium supplemented with the XFBD total recipe, XFBD herb extracts, or compounds for 24 h. For a single treatment, the concentrations were as follows: 0.25 μg·mL–1 for Ephedrae H., 1 μg·mL–1 for Pogostemonis H., 2.5 μg·mL–1 for Verbenae H., 6 μg·mL–1 for Artemisiae H., 34 μg·mL–1 for Polygoni R., 50 μg·mL–1 for Coicis S., and 200 μg·mL–1 for the other herbs. When testing cooperative effects, the dosages of each herb were reduced to half of that used for single treatment. For compound tests, the concentrations were 20 μmol·L–1 for atractylenolide I and 50 μmol·L–1 for all other compounds. The concentrations of herbal extracts and compounds were determined individually or in combination according to their toxicity and solubility, as well as our previous experiences [19]. The zebrafish caudal fin-wounding model has been reported previously [18–20]. In brief, the embryos were anesthetized in 0.016% Tricaine (A5040; Sigma–Aldrich) and subjected to tail amputation with a sterile scalpel at a consistent anatomic site, which was slightly posterior to the end of the tail circulation. The fish embryos were recovered in fresh embryo medium after tail transection for about 5 min, and then re-incubated in respective drug solution for 4 h before imaging.
《2.12. Microscope imaging and image analysis》
2.12. Microscope imaging and image analysis
The tail accumulations of macrophages or neutrophils were imaged using a fluorescent microscope (DMI3000B; Leica, Germany) and quantified with Image J software (version 1.52q; NIH, USA). The number of cells in a defined area of the same size in the wounded tail region was manually counted, as reported previously [20,21]. For time-lapse imaging and the automated tracking of macrophage migration, the amputated larvae were mounted in 1.2% low melting agarose (0815; Amresco, USA) in embryo medium with or without drug solution, on a scientific glass-bottom cell culture dish (F15mm; NEST, China). Confocal microscopy was performed using a confocal laser scanning microscope (TCS SP8, Leica) with a 10× photomultiplier tube (PMT) detector objective. Time-lapse microscopy was performed at 28 °C with an interval of 8 min. From the obtained z-stacks, aligned maximum projection images and videos were generated using Leica Application Suite X software. For velocity analysis, migrating macrophages were manually traced using Image J software.
《2.13. RNA isolation, complementary DNA (cDNA) synthesis, and quantitative polymerase chain reaction (qPCR)》
2.13. RNA isolation, complementary DNA (cDNA) synthesis, and quantitative polymerase chain reaction (qPCR)
The total RNA of the zebrafish embryos was extracted with an RNA-quick purification kit (RN001; EZ Bioscience, USA), and then converted to single-strand cDNA with a HiFiScript cDNA synthesis kit (CW2569M, ComWin Biotech, China). Real-time polymerase chain reaction (RT-PCR) was performed using the two-step quantitative RT-PCR method with 2× SYBR green qPCR mater mix (B21202; Bimake, China). The sequences of the primers are:  (internal reference) forward AGAAGGCTGCCAAGACCAAG, reverse AGAGGTTGGGAAGAACACGC;
(internal reference) forward AGAAGGCTGCCAAGACCAAG, reverse AGAGGTTGGGAAGAACACGC;  forward CGCTAAGGCAACTGGAAGAC, reverse CCAGACCACTGGGAAACACT;
forward CGCTAAGGCAACTGGAAGAC, reverse CCAGACCACTGGGAAACACT;  forward TGTGTGTTTGGGAATCTCCA, reverse CTGATAAACCAACCGGGACA.
forward TGTGTGTTTGGGAATCTCCA, reverse CTGATAAACCAACCGGGACA.
《2.14. Statistical analysis》
2.14. Statistical analysis
All data are presented as the mean ± standard deviation. Differences between the two groups were analyzed using the two-tailed Student’s t-test. Multiple group comparison was conducted by means of one-way analysis of variance (ANOVA). Data analysis was performed using GraphPad PRISM 8.0 software (GraphPad Software, Inc., USA). A P < 0.05 was considered to be statistically significant.
《3. Results》
3. Results
《3.1. Characterization of the chemical constituents in XFBD by highresolution MS》
3.1. Characterization of the chemical constituents in XFBD by highresolution MS
Despite its positive clinical feedbacks in COVID-19 treatment, the chemical composition of XFBD remains elusive, hindering any further elaboration of its pharmacological mechanism. Therefore, we first analyzed the chemical composition of XFBD by means of UPLC-Q-TOF-MS (Fig. 1(a)). After examining the raw data, 171 individual ion peaks were recognized as major compounds. From the results of the high-resolution m/z, the molecular formulas of these compounds were obtained. Their candidate structures were then tentatively deduced from the literature and public databases, and were further confirmed via MS fragmentations. Subsequently, a total of 104 constituents were identified or tentatively characterized for XFBD (Table S1 in Appendix A). The identified compounds included 36 flavonoids, 22 terpenes, 21 carboxylic acids, 3 alkaloids, 3 anthraquinones, and 19 other types. Among them, 33 compounds were unambiguously identified through comparisons with reference standards in terms of retention time and mass spectra (Fig. S3 in Appendix A).
According to the similarity of MS2 spectra, the molecular networking of XFBD was constructed using the MS2 data (Fig. 1(b)). Every single ion with MS2 data recognized from the raw data was represented as a node. Based on the MS spectrum of the negative mode, 1125 nodes and 1686 links were retrieved, with 23 clusters having more than five nodes, while 616 nodes and 1272 links were obtained from the MS spectrum of the positive mode, with nine clusters contain more than five nodes (Fig. S4 in Appendix A). Consistent with the MS identification results, flavonoids, terpenes, and carboxylic acids constituted the main clusters in molecular networking, providing evidence that these compounds are major components of XFBD.
《Fig. 1》
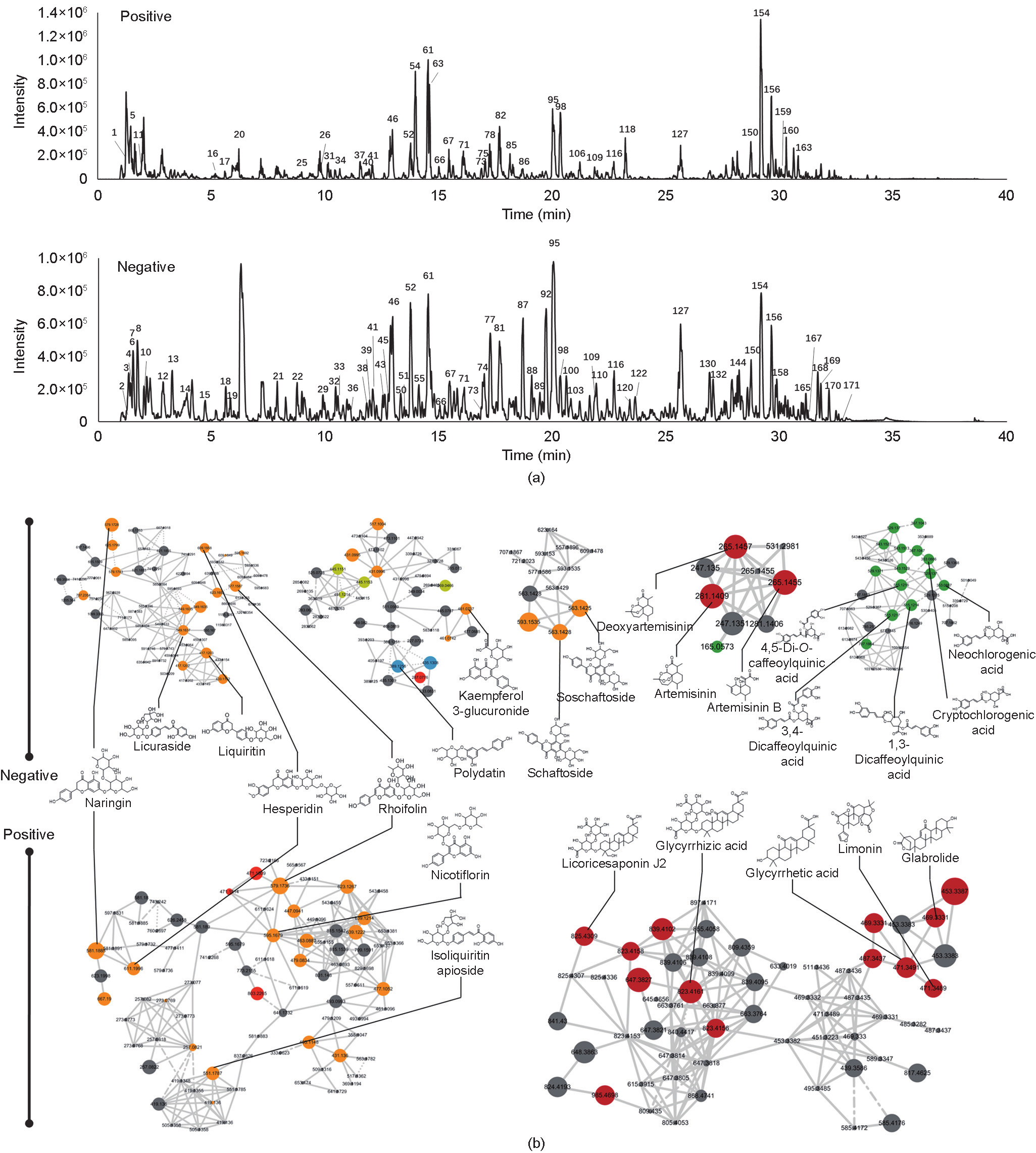
Fig. 1. MS-based molecular networking and network pharmacology analysis of XFBD. (a) Base peak mass spectrum of XFBD with positive and negative modes. (b) The main clusters of molecular networking of XFBD. Nodes with different colors represent different compound classifications. Yellow: flavonoid; red: terpenes; green: carboxylic acids; blue: glycosides.
《3.2. MS-based network pharmacology analysis suggests XFBD plays regulatory roles in viral infection, inflammation, and lung injury》
3.2. MS-based network pharmacology analysis suggests XFBD plays regulatory roles in viral infection, inflammation, and lung injury
Next, the potential functional targets of the LC-MS-identified XFBD compounds were analyzed by network pharmacology. The targets of the identified compounds were obtained by searching in databases (e.g., ETCM, TCMSP, and TargetNet), and a total of 751 hits were predicted as XFBD targets, with over 33.5% of the hits being recognized as COVID-19-associated genes (see Section 2.5 for details). We continued to use these overlapping genes to construct a target network. In the network, 2897 connections were established among 245 hits, with the average connectivity of 23.6 as network topological feature. (Fig. S5 in Appendix A). Next, functional enrichment analysis was performed toward the hub targets by means of STRING. Remarkably, 17% of the enriched pathways were related to lung injury, 13% were related to bacterial or virus infection, and 12% were related to immunoregulation (Fig. S6 in Appendix A). The integrated relationship between the compounds–targets-pathway of antivirus activity, immune regulation, and anti-lung injury activity is presented in Fig. S7 in Appendix A. It is notable that the Toll-like receptor (TLR) and nucleotidebinding oligomerization domain (NOD)-like receptor signaling pathways—the two major pattern-recognition receptors that are critical in activating immune responses toward microbial infections [22]—are among the top pathways related to XFBD, with target genes such as TNF-α, phosphatidylinositol-4,5-bisphosphate 3- kinase catalytic subunit c gene (PIK3CG), and prostaglandinendoperoxide synthase 1/2 gene (PTGS1/2).
Taken together, the results from MS-based network pharmacology strongly suggested that XFBD has a regulatory role in immune response to pathogen invasions.
《3.3. XFBD inhibits LPS-induced acute inflammation in mice》
3.3. XFBD inhibits LPS-induced acute inflammation in mice
In order to validate the predicted role of immune regulation of XFBD by network pharmacology analysis, we tested the function of XFBD in the LPS-induced inflammation mice model. LPS administration at 20 mg·kg–1 for 4 h was sufficient to induce acute inflammation in mice, as demonstrated by greatly increased serum levels of proinflammatory cytokines, including IL-6, TNF-α, and IL-1β. It was found that pretreatment with XFBD for 3 days before LPS stimulation significantly inhibited the release of proinflammatory cytokines, suggesting an inhibitory role of XFBD in LPS-induced immune hyperactivation (Figs. 2(a)–(d)). Moreover, histopathological examination detected typical inflammation changes in the lung tissue after LPS treatment (Fig. 2(e)). Compared with the uninjured control group, the alveolar septa of the LPS-stimulated mice were dramatically thickened, with severe inflammatory cell infiltration. The pulmonary pathological alterations were largely attenuated in the XFBD pretreated mice, indicating a protective effect of XFBD on LPSinduced lung injury. The pathological images were further quantified for the area of interstitial pulmonary tissue versus the airfilled alveolar spaces. The results suggested thickened alveolar septa in the LPS-treated mice, indicating increased inflammatory infiltration; this phenotype was largely resolved in the mice with XFBD treatment (Fig. 2(f)).
《Fig. 2》
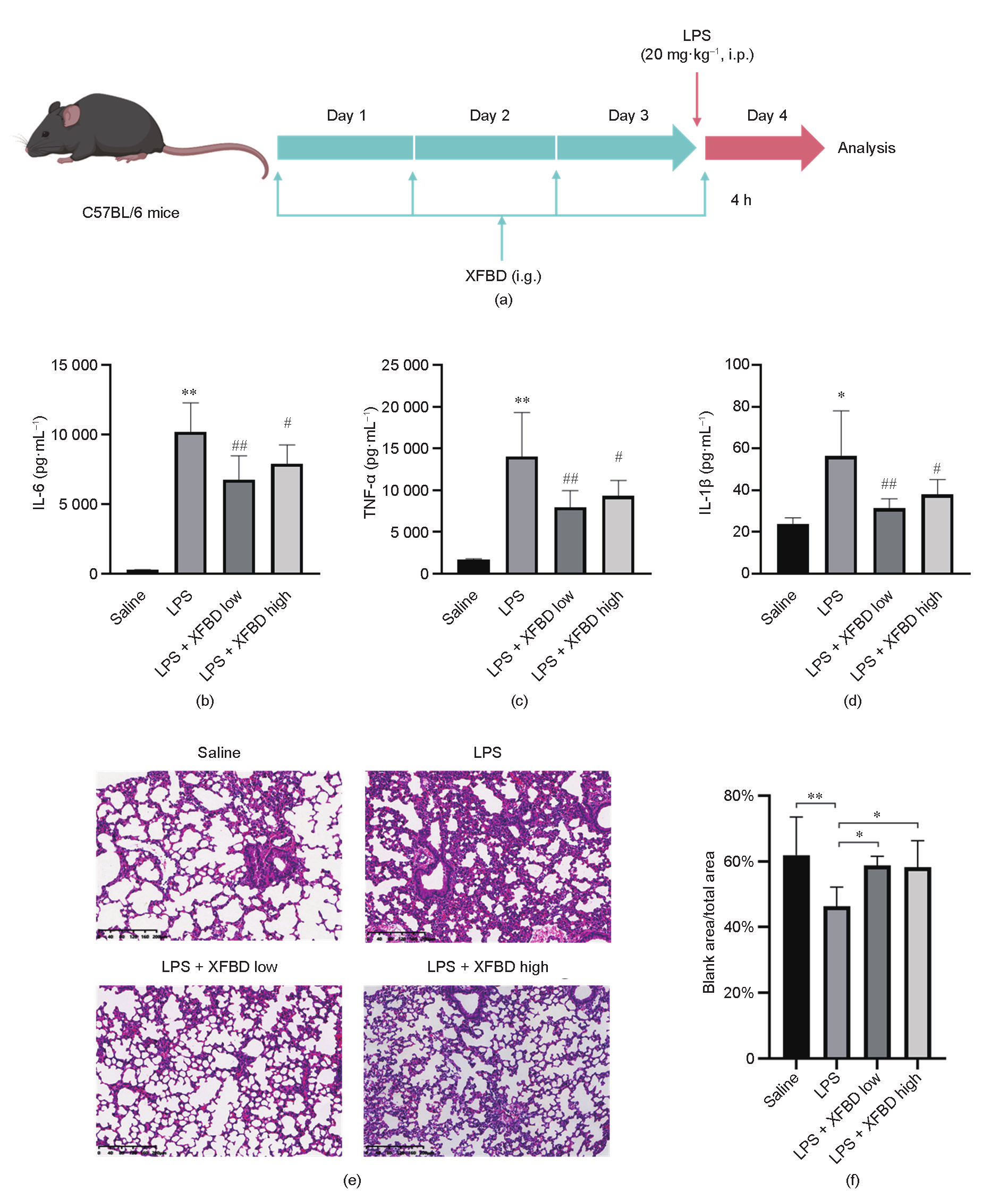
Fig. 2. XFBD inhibits LPS-induced acute inflammation in mice. (a) Scheme of XFBD administration and LPS injection. (b–d) Effects of XFBD (high: 4.32 g·kg–1 ; low: 2.16 g·kg–1 ) on inflammatory cytokines secretion in serum induced by LPS (20 mg·kg–1 for 4 h). At least six animals were examined in each group (*: P < 0.05, **: P < 0.01 vs saline; #: P < 0.05, ##: P < 0.01 vs LPS). (e) H&E staining of the lung tissue of different groups. (f) Quantification of mice lung pathological sections: (size of blank area/size of total area) × 100%. For analysis, an image of one section was taken for each mouse and three mice were included in each group (*: P < 0.05, **: P < 0.01 vs saline). i.p.: intraperitoneal injection; i.g.: intragastrical administration.
In order to analyze the endogenous constituents and metabolites of XFBD, LC-Q-TOF-MS analysis was performed for the serum and lung samples of LPS-stimulated mice after the oral administration of XFBD (Fig. S8 in Appendix A). According to the results, 67 constituents were detected in the serum, with 1 constituent existing only as prototypes, 20 existing as both prototype constituents and metabolites, and 46 only existing as metabolites. A total of 57 constituents were detected in the lung, with 2 presenting as prototypes, 21 as both prototype constituents and metabolites, and 34 as only metabolites. Quantitative analysis further showed that several representative compounds—including glycyrrhizic acid, naringin, and isoliquiritin—were present in the serum or lung samples of the LPS-treated mice after 3 days of oral administration of XFBD, at concentrations in the range of 0.4–35.0 ng·mL–1 . Moreover, higher concentrations of several samples were observed in the lung sample as compared with the serum , suggesting that the lung may be one of the key target organs for XFBD. The distribution of the representative compounds is shown in Fig. S9 in Appendix A, and detailed information is provided in Tables S2 and S3 and Fig. S10 in Appendix A.
《3.4. XFBD regulates gene expression in multiple infection- and inflammation-related pathways》
3.4. XFBD regulates gene expression in multiple infection- and inflammation-related pathways
In order to systematically evaluate the impact of XFBD treatment on the gene expression profile in the LPS-stimulated mice model, PBMCs were collected and examined by means of messenger RNA (mRNA) sequencing. Based on a transcriptomic analysis of LPS-induced mice with or without XFBD treatment, 1077 differentially expressed genes (DEGs) were identified, with 871 upregulated and 206 downregulated genes (Fig. 3(a)). Moreover, 357 DEGs were differentially expressed between the control group and the LPS-only group, which may be related to the development of LPS-induced inflammation (Fig. 3(b)).
We next included the 357 DEGs in the analyses of PPI (Fig. S11 in Appendix A) and pathway enrichments. Platelet activation, extracellular matrix (ECM)–receptor interaction, and leukocyte transendothelial migration were among the top enriched pathways, which are all key processes in immune response (Fig. 3(c)). Moreover, among the 357 DEGs, the expression of 107 DEGs were reversed back or partially rescued to normal levels after XFBD treatment (Fig. S12 in Appendix A). Importantly, the expression of multiple key regulators in the process of inflammation was changed after XFBD treatment. For example, vascular endothelial growth factor A (VEGFA), which has been suggested to promote inflammation during SARS-CoV-2 infection [23], was identified as the target with the most connected targets and can be downregulated by XFBD. It was particularly noticeable that multiple hits among the regulated genes have been reported to play a role in macrophage functions. For example, soluble CD93 (sCD93) was reported to induce the differentiation of monocytes to macrophage-like cells [24], and an increase in sCD93 was related to macrophage infiltrating [25]. Moreover, long isoforms of nuclear factor erythroid 2-related factor 1 (NFE2L1) function as a negative regulator of M1 polarization, which is the inflammatory phenotype of macrophages, and proinflammatory response [26]. Since macrophages are potent immunoregulatory cells in the innate immune response, we wondered whether XFBD works by mediating the function of macrophages. Therefore, we continued to test the effects of XFBD on macrophage activation and migration, the two key processes for macrophage-mediated immune response.
《Fig. 3》

Fig. 3. Transcriptomic analysis suggested that XFBD regulates gene expression in multiple infection- and inflammation-related pathways. (a) Counts and distribution of DEGs of LPS-stimulated mice with or without XFBD treatment. Red: upregulated genes; green: downregulated genes; blue: genes without significant changes. Padj: adjust P value. (b) Venn diagram of DEGs in respective groups. (c) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment of 357 overlapping DEGs. FC: fold change.
《3.5. XFBD and its active compounds inhibit macrophage activation in vitro》
3.5. XFBD and its active compounds inhibit macrophage activation in vitro
As macrophage activation plays a critical role in the initiation of inflammation, we examined the effects of XFBD on a macrophage-activation cell model stimulated with the TLR2 agonist Pam3CSK4 and the TLR3 agonist Poly(I:C) [27]. Previous studies have suggested that—in contrast to individual TLR activation— multi-TLR activation may stimulate more severe cytokine response in macrophages [28]. Since IL-6 is a major proinflammatory cytokine secreted by macrophages and is associated with the differentiation of M1 macrophages [29], the release of IL-6 was used to evaluate the level of macrophage activation in our assay. As expected, TLR agonist dramatically induced the release of IL-6 in our model (Figs. 4(a) and (b)). Importantly, pretreatment with XFBD in the range of 6.25–100.00 μg·mL–1 significantly inhibited IL-6 release in a dose-dependent manner (Fig. 4(b)). Subsequently, the anti-inflammatory effects of all 12 herbal extracts in the XFBD formula were assessed at dosage levels determined by respective cytotoxicity assays. As a result, Polygoni R., Phragmitis R., Citri grandis E., Glycyrrhizae R., and Coicis S. strongly inhibited the release of IL-6 in a dose-dependent manner, while Armeniacae S. exerted certain effects on IL-6 release (Fig. 4(c)). We further screened the representative compounds of these effective herbs. Isoliquiritin, acteoside, and polydatin showed a dosedependent tendency to inhibit IL-6 secretion at 10, 20, and 50 μmol·L–1. Among these, statistically significant inhibitory effects were observed for isoliquiritin and acteoside at 50 μmol·L–1 (Fig. 4(d)). Previous studies have suggested that the mitogen-activated protein kinase (MAPK) family, including p38 MAPK, c-Jun N-terminal kinase (JNK), and MAPK-extracellular signal regulated kinase (ERK) (MEK), plays essential roles in modulating inflammatory responses mediated by macrophages and other immune cells [30,31]. Therefore, we further explored the impact of XFBD compounds on MAPK signaling, using isoliquiritin as a representative. Interestingly, isoliquiritin dramatically downregulated the protein level of phosphorylated JNK but showed an enhancing role in the phosphorylation of p38 (Figs. 4(e) and (f)). A negative regulatory role of p38 on JNK phosphorylation has been consistently reported [32], and JNK hyperactivation is correlated with the M1 phenotype switch of macrophages [33]. In combination, these results validated the in vitro inhibitory effects of XFBD and its major components on the process of macrophage activation.
《Fig. 4》
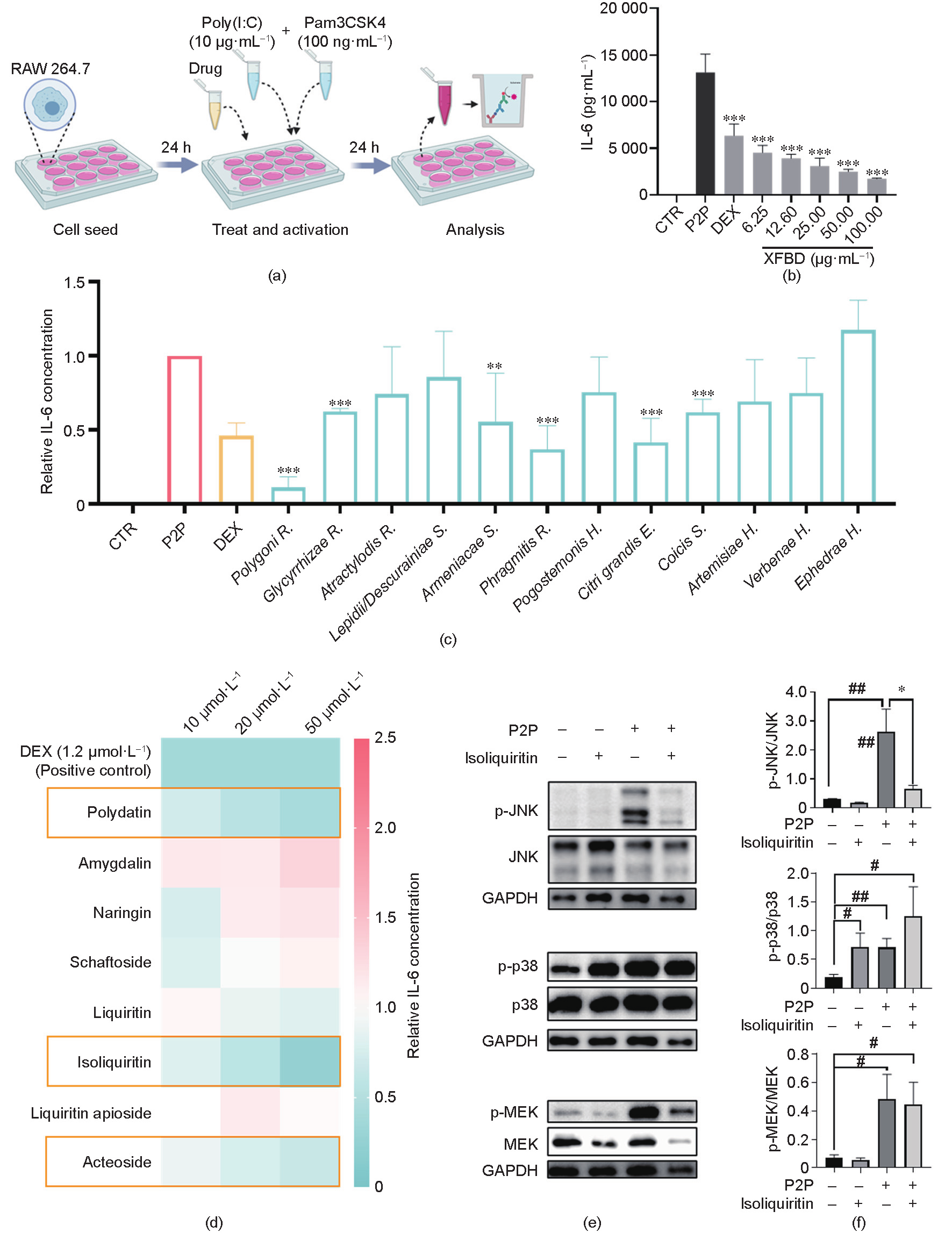
Fig. 4. XFBD and its active compounds inhibited macrophage activation in vitro. (a) Scheme of macrophage activation and analysis. (b) XFBD extracts of 6.25–100.00 μg·mL–1 inhibited IL-6 release on the macrophage-activation model in a dose-dependent manner after 24 h of treatment (n = 3, ***: P < 0.001 vs P2P). (c) Protein level of IL-6 in macrophage cells with treatment with different compounds (n = 3, **: P < 0.01, ***: P < 0.001 vs P2P). DEX was used as the positive control. The detailed concentrations of each treatment are listed in the Section 2.9. (d) The effects on IL-6 release of representative XFBD compounds with different doses in the P2P-stimulated macrophage model. The relative IL-6 concentrations were calculated by comparing the value of each compound-treated group with those of the model group. (e, f) Effect of isoliquiritin (50 μmol·L–1) on the phosphorylation of JNK (p-JNK), p38 (p-p38), and MEK (p-MEK). Representative western blots are shown in (e) and statistical graphs are shown in (f) (n = 3; *: P < 0.05 vs P2P; #: P < 0.05, ##: P < 0.01 vs control). CTR: control.
《3.6. XFBD inhibits inflammation-induced macrophage recruitment in vivo》
3.6. XFBD inhibits inflammation-induced macrophage recruitment in vivo
The recruitment of immune cells from the microcirculation to the damaged tissue is a prerequisite step for subsequent inflammatory reactions, and thus is a key pathological process to target in hyperinflammation treatment. Therefore, we continued to examine the function of XFBD and its components on the process of macrophage mobilization. In order to dynamically observe the endogenous process of macrophage migration, we employed a zebrafish inflammation model induced by tail amputation. As observed in previous studies [20], accumulation of fluoresceinlabeled macrophages and neutrophils was detected at 4 h post amputation (hpa) in the Tg (mpeg: eGFP) and Tg (Lyz: dsRED2) transgenic lines, respectively. To examine its effects, XFBD was supplemented in the culture medium of zebrafish embryos both 24 h before and immediately after tail amputation until microscopic analysis (Figs. 5(a) and (b)). Importantly, XFBD displayed a dose-dependent effect in decreasing the number of macrophages at the wounding site at 4 hpa (Figs. 5(c) and (d)). Moreover, the negative regulation of XFBD on cell migration seems to vary according to the type of innate immune cells, since the accumulation of neutrophils at the transected tails was not influenced by XFBD treatment (Figs. 5(e) and (f)). In sum, these results suggested that XFBD selectively inhibits inflammation-induced macrophage accumulation in the zebrafish tail amputation model.
Next, using time-lapse confocal imaging, the migration process of macrophages after tail transection was dynamically recorded. It was noticeable that the moving velocity of macrophages toward the wounding sites was greatly reduced in the embryos incubated in XFBD, which indicated that XFBD directly suppressed macrophage mobilization (Figs. 5(g) and (h), Videos S1 and S2 in Appendix A). Moreover, to assess the drug impacts on the inflammation status of the tail-amputated embryos, we examined the transcriptional levels of IL-6 and IL-1β. The expressions of both proinflammatory cytokines were found to be significantly attenuated upon XFBD treatment in the tail-amputated embryos (Fig. 5(i)).
《Fig. 5》

Fig. 5. XFBD inhibits inflammation-induced macrophage recruitment in zebrafish. (a) Illustration of the timeline of drug protection and tail amputation. Red dotted line represents the amputation site, and the blue box represents the region for the cell counting of recruited inflammatory cells. (b) Toxicity effects of different doses of XFBD (50–800 μg·mL–1 ) on fish survival and general development. (c) Representative images and (d) quantification of the accumulation of macrophages at the wounding sites of the control or the XFBD-treated (50–200 μg·mL–1 ) embryos. (e) Representative images and (f) quantification of the accumulation of neutrophils at the wounding sites of the control or the XFBD-treated (50–200 μg·mL–1 ) embryos. (g) Time-lapse imaging of macrophage movement in the control and the XFBD-treated (200 μg·mL–1 ) embryos at indicated times after tail amputation. (h) Quantification of the moving velocity of macrophages. (i) Gene expression of cytokines IL-6 and IL-1β in tail-amputated embryos with or without XFBD (200 μg·mL–1 ) treatment. In (c, e, g), white dotted lines outline the fish embryos; yellow (c, e) or red (g) dashed lines mark the transection site; yellow and red asterisks (g) mark the moving trajectory of the representative macrophages. mpa: minutes post amputation; UC: tail-uncutted embryos; CTR: tail-cutted embryos without treatment; XFBD group: tail-cutted embryos with XFBD treatment of different dosages. *: P < 0.05; **: P < 0.01.
《3.7. Multiple XFBD herbal components showed positive cooperative effects in inhibiting microphage migration 》
3.7. Multiple XFBD herbal components showed positive cooperative effects in inhibiting microphage migration
In addition, we considered whether any cooperative effect exists in the XFBD formula in downregulating microphage migration. A total of 12 herbal extracts were prepared from the total XFBD solution, and the regulatory effect of each extract on microphage migration was screened. Artemisiae H. and Ephedrae H. significantly inhibited the recruitment of macrophages to the wounding site. Atractylodis R. also displayed a marginal inhibitory effect yet without statistical significance (Fig. 6(a)).
The pathological process of inflammation is related to the symptoms of ‘‘hotness” and ‘‘toxicity,” as described in the TCM theory. Therefore, herbs with a documented function of ‘‘Qing Re Jie Du” (clearing heat and detoxicating) were selected from the XFBD total recipe and further examined for their compatibility with Artemisiae H. and Ephedrae H. In order to determine the cooperative effect, the concentrations of most herb extracts were reduced to half in the combinations, as compared with each single administration. Indeed, the combination of Artemisiae H. and Ephedrae H. showed a superior effect in comparison with that of either herb alone. Moreover, when combined with Atractylodis R. or Polygoni R., Ephedrae H. displayed a strikingly increased effect in inhibiting macrophage migration, which was much improved in comparison with Ephedrae H. alone at doubled concentration. A significant inhibitory effect on macrophage migration was also observed in the combination of Ephedrae H. and Citri grandis E. (Fig. 6(b)). Taken together, these findings strongly support the existence of cooperative effects among different herbal components in the XFBD recipe.
Finally, we searched for the active compounds responsible for the negative regulation of XFBD on wounding-induced macrophage migration. Based on the results of the herbal extracts screening, nine representative compounds in effective XFBD herbs were selected and tested in the zebrafish tail-amputation model (Figs. 6(c) and (d)). Notably, ephedrine and atractylenolide I, which are essential compounds in Ephedrae H. and Atractylodis R., respectively, showed significant effects in inhibiting macrophage migration to the transected tails of the fish embryos. In addition, kaempferol, a common compound detected in multiple XFBD herbs including Artemisiae H., Ephedrae H., and Polygoni R., largely reduced the number of accumulated macrophages at the wounding sites.
《Fig. 6》

Fig. 6. Screen for active herbs, herbal combinations, and compounds of the XFBD recipe in macrophage mobilization. Quantification of the number of macrophages accumulated at the wounding site of embryos treated with (a) different XFBD herb extracts, (b) herb combinations, and (c) compounds. The dosages of each treatment are listed in the Sections 2.9 and 2.11. **: P < 0.01. Groups labeled without a common letter were significantly different (P < 0.05) in (b). (d) Molecular structure of compounds with significant effects in regulating macrophage recruitment. (e) Model of the regulatory effects of XFBD and its components on macrophage-mediated inflammation response. Red boxes highlight the active herbs identified from the macrophage activation model in P2P-stimulated RAW 264.7 cells; blue boxes highlight active herbs identified from the macrophage mobilization model in caudal fin-wounding zebrafish. CTR: tail-cutted embryos without treatment.
《4. Discussion》
4. Discussion
Although numerous medications have been developed against COVID-19, the therapeutic outcome remains unsatisfying. For example, shortened hospitalization time was observed in COVID19 patients treated with the anti-Ebola drug remdesivir, yet no significant impact on mortality rate was reported [34]. For multiple other drugs, including hydroxychloroquine (an anti-malaria drug) [35], lopinavir–ritonavir (an anti-HIV cocktail) [36], and azithromycin (antibiotic) [36–38], no clinical benefits were observed.
SARS-CoV-2 infection leads to dysfunctional immune responses, accompanied by acute respiratory distress syndrome (ARDS). When these are detected in critical patients with COVID-19 [39], the direct result is respiratory failure. Cytokine storm is considered to be one of the major causes of ARDS [40]. In a subset of COVID-19 patients, features of systemic hyperinflammation have been observed, which may be caused by cytokine storm or macrophage overactivation. Low doses of corticosteroids can downregulate proinflammatory cytokine expression and accelerate the resolution of systemic inflammation in pneumonia; yet they may also reduce pathogen clearance and increase viral replication [41,42]. Thus, the usage of corticosteroids in COVID-19 treatment is still under debate [43]. Preliminary results from the Randomised Evaluation of COVID-19 Therapy (RECOVERY Trial) suggested that DEX reduces 28-day mortality in COVID-19 patients with severe respiratory complications. However, no benefit was found in patients who were not receiving respiratory support after DEX treatment [39].
Pathological studies detected that macrophages and monocytes are the most abundant group among infiltrated immune cells in the alveoli of COVID-19 patients [44]. Macrophages are essential for the effective control of infections, with their three essential biological functions of phagocytosis, antigen presentation, and cytokines production [45]. Two major subpopulations have been identified in macrophages: classically activated or inflammatory M1 macrophages; and alternatively activated or antiinflammatory M2 macrophages [46]. M1 macrophages are induced by diverse pathological stimuli, such as trauma or infections; they then actively migrate to the injured sites and help to eliminate the stimulus signal through cytokines secretion and cytotoxic functions. However, excessive macrophage activation may also contribute to hyperinflammation and cytokine storm. Highly elevated C-reactive protein (CRP) and cytokine profiles were also reported in COVID-19 patients, including IL-1β, IL-2, IL-6, IL-17, IL-8, TNF-α, and C–C motive chemokine ligand 2 (CCL2) [47]. Human monocytes/macrophages are the major producers of IL-1β and IL-6 [48], with the former further enhancing the production of the latter. Among these proinflammatory cytokines, IL-6 in particular has been shown to decrease natural killer cell cytolytic function, resulting in prolonged interactions between innate and adaptive immune cells and eventually increased cytokine cascade [49]. Therefore, the concentration of IL-6 in peripheral blood has been used to assess the severity of systemic cytokine responses in patients [50]. Antagonism therapy of IL-6 (and some other cytokines including IL-1) is undergoing development in the treatment of COVID-19 patients with immune dysregulation.
In China, in addition to the abovementioned medications, TCM has been widely used to treat COVID-19. More than 74 000 patients were treated with TCM, and an overall effective rate of over 90% was suggested by clinical observation in multiple healthcare centers, as demonstrated by a significantly shortened total recovery time and improved disease prognosis [51,52]. According to the TCM theory, the composition of XFBD plays a role in regulating immune responses, dampening inflammation, regulating hepatic and biliary metabolism, and supporting the regular function of the lung [6]. However, the underlying pharmacological mechanism of XFBD remains unknown, largely due to its extremely complicated components, in comparison with most chemical drugs. Facing this tough challenge, we proposed a multimodal research strategy for the identification of TCM effective components, which combined the advantages of various biological and chemical techniques and model systems. Based on an unbiased analysis of MSbased network pharmacology, the regulatory effects of XFBD were found to be oriented to immune function and inflammation. Next, using a classical mice inflammation model, we validated the antiinflammation effects of XFBD. The transcriptomic assay further suggested that many genes with altered expression after XFBD treatment were related to macrophage-mediated immune response and leukocyte migration. Consequently, an in vitro cell model and an in vivo zebrafish model were employed to systematically screen the effective components in the XFBD formula. Remarkably, we identified the roles of different XFBD herbs in the regulation of two highly related pathological processes—macrophage activation and macrophage migration—in the development of immune responses (Fig. 6(e)).
Several herbs exhibited excellent activity in inhibiting macrophage activation, especially Polygoni R., Phragmitis R., and Citri grandis E. In particular, Polygoni R. is an effective herbal medicine in the treatment of inflammation and infection [53]. Our experimental results also indicated its important anti-inflammatory role. Phragmitis R. is another multi-functional herb. Stigmasta-3,5-dien7-one, a chemical compound isolated from Phragmitis R., has been reported to reduce nitric oxide (NO), prostaglandin E2, and the proinflammatory cytokine level of LPS-induced macrophages through the nuclear factor (NF)-κB pathway [54]. Citri grandis E. is a classical herbal medicine with antitussive, expectorant, and anti-inflammatory effects [55,56], all of which are related to its beneficial effects in the treatment of respiratory disease.
Moreover, apparently positive cooperative effects were observed in multiple XFBD herbs, especially among Artemisiae H., Ephedrae H., Atractylodis R., and Polygoni R., in regard to their inhibition of macrophage migration. According to the TCM theory, Artemisiae H. and Polygoni R. have the function of clearing hotness and toxins in the body (‘‘Qing Re Jie Du”), which is partially parallel to the process of anti-inflammation in modern medicine. Furthermore, Ephedrae H. has a documented effect in reducing fever and relieving respiratory distress, whereas the major function of Atractylodis R. lies in dispersing cold. A combination of these herbs, as well as other XFBD herbs, showed extra effectiveness in treating COVID-19 patients with ‘‘Shi Du Yu Fei” (the lung is stagnated by noxious dampness) symptoms. Although it is still difficult—and not appropriate—to explain the TCM theory completely from the perspectives of modern medicine and biological sciences, our observations of the positively cooperative effects among these herbs robustly support the compatibility theory of TCM.
Finally, through the compounds screening, we identified six XFBD compounds that are active in suppressing macrophage activation or mobilization. Polydatin is a multi-effect compound used for relieving cough, regulating blood lipids, lowering cholesterol, and resisting shock, with reported roles in inhibiting NO and prostaglandin E2 (PGE2) production, as well as NF-κB activation and MAPKs phosphorylation in LPS-induced RAW 264.7 macrophages [57]. Isoliquiritin has been suggested to promote wound healing through macrophage recruitment and inflammatory response [58], and to inhibit LPS-induced inflammatory responses in murine macrophages by suppressing the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) [59]. Interestingly, it was reported that isoliquiritin suppressed the phosphorylation of inhibitor of NK-κB kinase (IKK), ERK1/2, and p38 in LPStreated RAW 264.7 macrophages, whereas the phosphorylation of JNK1/2 was unaffected [60]. However, our results indicated that isoliquiritin downregulated JNK1/2 phosphorylation on Poly(I:C)/ Pam3CSK4-induced RAW 264.7. One possible explanation is that the anti-inflammatory mechanisms induced by different TLRs agonists could be different.
Ephedrine is an adrenomimetic drug with known effect in relaxing the bronchial smooth muscles. In addition, ephedrine hydrochloride has been reported to negatively regulate the production of proinflammatory cytokines in an LPS-stimulated mice model [61]. Nevertheless, the usage of ephedrine is illegal or strictly controlled in many regions, since it is a major ingredient of methamphetamine. A wide spectrum of pharmacological activities has been identified for atractylenolide I and kaempferol, including anti-inflammation, antitumor, and antioxidant properties. In an LPS-induced mice sepsis model, reduced levels of proinflammatory cytokines (including IL-1β and IL-6) and an increased survival rate were observed in atractylenolide I-treated mice [62]. As a low-cost natural dietary flavonol, kaempferol is widely used in the treatment of diverse chronic and acute inflammations [63].
Nevertheless, our study has some limitations. With the aim of evaluating the multi-target effects of the complicated TCM formula of XFBD, we screened the drug effects of XFBD and of the herbs/compounds that comprise XFBD using multiple inflammation-related models. Nevertheless, an in-depth investigation of the molecular mechanism of XFBD or of its active compound on particular pathways is still lacking and is warranted for future studies. Furthermore, although we employed the approaches of network pharmacology and transcriptomics analysis to generate a big picture of the possible roles of XFBD, further biological studies are needed to validate the findings of these in silico results in future works. Moreover, it was noticeable that, although multiple XFBD compounds were detected in the mice serum and lung tissue, their concentrations were very low compared with the effective concentrations in the in vitro models. One possible reason is that the metabolites of these compounds may also play important regulatory roles in the endogenous environment. In addition, the concentrations required for synergistic cooperation among different effective compounds may be substantially lower than the required effective concentration for each of them, as compared with the in vitro assays. Since a typical TCM formula such as XFBD includes a large number of compounds, each of which may generate dozens or even hundreds of metabolites after entering the human body, it is very difficult to experimentally test these hypotheses. More convenient large-scale drug screening that includes multiple compound combinations in endogenous model systems or organ chips may help to unravel this question.
《5. Conclusions》
5. Conclusions
The present study identified the negative regulatory roles of XFBD, a first-line TCM for COVID-19 treatment, in inflammationinduced microphage activation and mobilization. Through multimodal research approaches including MS-based network pharmacology, transcriptomic analysis, and multiscale bioassays, single herb, herb combinations, and compounds with active effects in inhibiting macrophage-mediated immune responses were identified from the XFBD formula. These results illustrate a key pharmacological mechanism of XFBD in inflammation regulation, which may help to hint at the therapeutic effect of XFBD in COVID-19 treatment.
《Acknowledgments》
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2020YFA0708004), the National Natural Science Foundation of China (81822047 and 31971088), and the Foundation of State Key Laboratory of Component-based Chinese Medicine (CBCM2020104). Yi Wang was supported by the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-D202002). The authors are grateful for the support from ZJU PIIMolecular Devices JOINT LABORATORY.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Lu Zhao, Hao Liu, Yingchao Wang, Shufang Wang, Dejin Xun, Yi Wang, Yiyu Cheng, and Boli Zhang declare that they have no conflict of interest or financial conflicts to disclose.
《Appendix A. Supplementary data》
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eng.2021.09.007.












 京公网安备 11010502051620号
京公网安备 11010502051620号




