《1. Introduction》
1. Introduction
In recent years, phototherapy has received a great deal of attention due to its advantages of spatiotemporal addressability, minimal invasiveness, and relative clinical safety [1,2]. As early as 1903, Niels Ryberg Finsen—the founder of modern phototherapy—was awarded the Nobel Prize for treating a skin disease (lupus vulgaris) using sunlight and ultraviolet (UV) radiation [3,4]. Since then, phototherapy has been continuously developed in biomedical engineering to combat skin diseases (e.g., eczema, psoriasis, and itchy skin) and mood and sleep disorders (e.g., seasonal affective disorder [5,6], non-seasonal depression [7], and circadian rhythm sleep disorders [8]). With the aid of phototherapeutic agents, such as photosensitizers and photothermal conversion agents (PTCAs), phototherapy has also proved to be an effective approach to ablate tumors [9].
Light sources that are commonly applied in cancer phototherapy include UV light (100–400 nm), visible light (400–760 nm), and near-infrared (NIR) light (760–1350 nm). However, due to the absorption of biological tissues, the widely used UV light and visible light have limited tissue penetration depth [10], which restricts traditional phototherapy to superficial tumors and may lead to the risk of relapse and metastasis [1,11]. In addition, low targeting efficiency caused by the nonspecific distribution of phototherapeutic agents is an important issue that must be addressed. Untargeted delivery of phototherapeutic agents in therapy procedures will not only lead to low bioavailability and poor efficacy, but also cause side effects.
With the development of nanotechnology, various phototherapeutic nanoagents based on versatile nanomaterials have been exploited to overcome the abovementioned limitations in cancer phototherapy [12–14]. Nanoagents with a specific size range (20–200 nm) accumulate in tumor tissue due to the enhanced permeation and retention (EPR) effect [15–17]. Therefore, nanomaterials hold potential to serve as carriers to deliver therapeutic molecules such as photosensitizers, PTCAs, drugs, and genes to tumor tissue, thereby achieving targeted therapy and combination therapy [18–20]. In order to address the issue of limited light penetration depth in phototherapy, NIR-light-responsive nanomaterials have been developed. For example, PTCAs that can directly absorb NIR light and generate heat have been used for NIR-triggered photothermal therapy (PTT). Upconversion nanoparticles (UCNPs), which can absorb long-wavelength NIR light and emit specific short-wavelength UV or visible light, have been used as a transducer to realize deeper penetration [21,22]. Thus, light-responsive nanomaterials offer the possibility of overcoming the obstacles in and expanding the applications of phototherapy.
In this review, we summarize recent advances in lightresponsive nanomaterials for cancer therapy (Table 1 [23–47]). These nanomaterials can be applied in PTT, photodynamic therapy (PDT), light-responsive molecule delivery, and light-controlled combination therapy (Fig. 1). We also discuss current challenges and prospects in phototherapy. The purpose of this review is to emphasize the significant role of light-responsive nanomaterials in cancer phototherapy and to provide further directions to expand their applications.
《Table 1》
Table 1 Summary of light-responsive nanomaterials in cancer therapy.
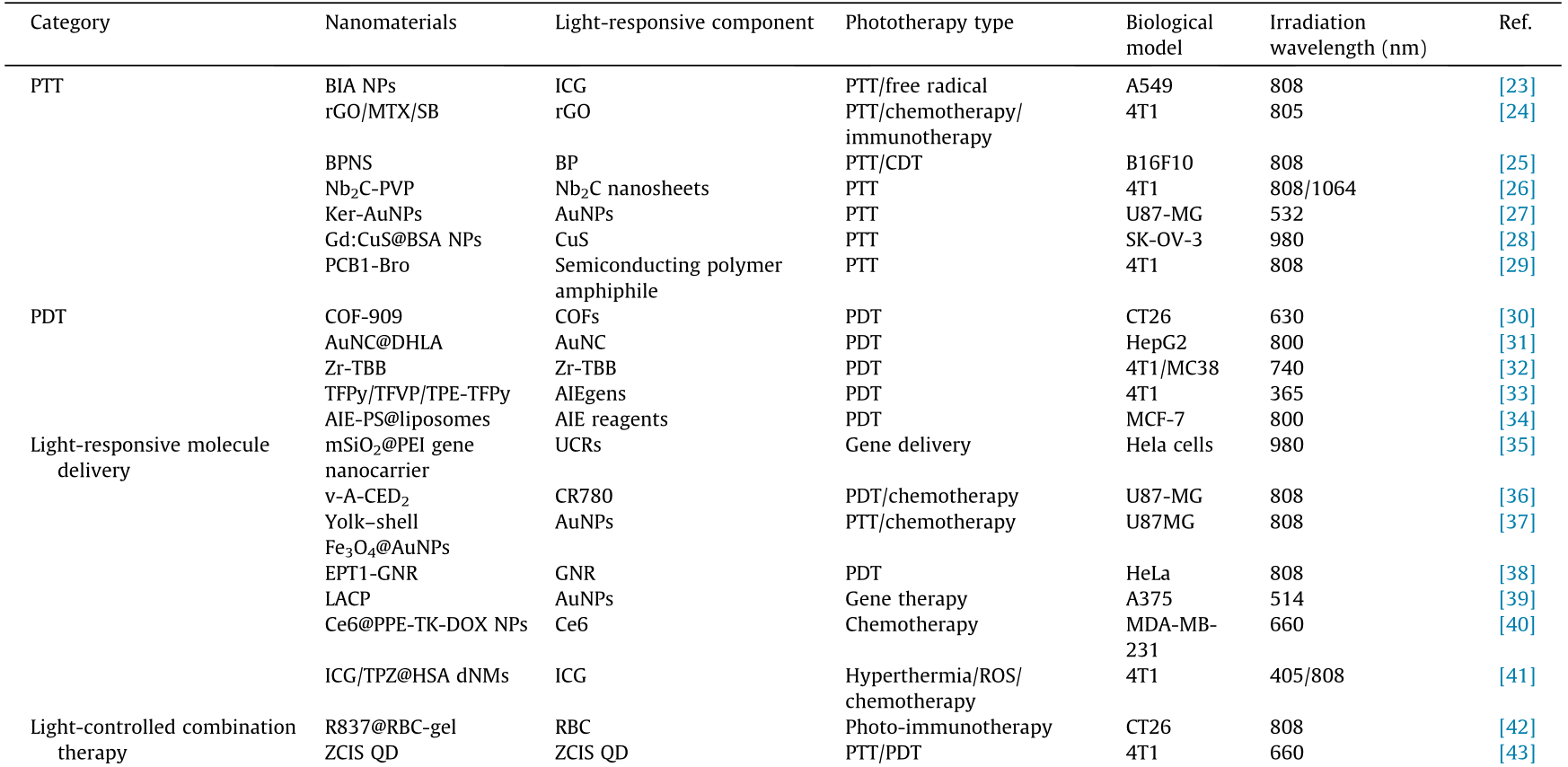

BIA: hybrid bovine serum albumin (BSA)–indocyanine green (ICG)-oxygen-independent radicals generator (AIPH); rGO/MTX/SB: mitoxantrone (MTX) and transforming growth factor beta (TGF-β) inhibitor SB-431542 (SB) co-loaded reduced graphene oxide (rGO); BPNS: a superior photothermal agent (PTA) based on black phosphorus (BP) nanosheets; CDT: chemodynamic therapy; Nb2C-PVP: a two dimensional niobium carbide (Nb2C) MXene nanosheet modified with polyvinylpyrrolidone (PVP); Ker-AuNPs: keratin-coated gold NPs (AuNPs); Gd:CuS@BSA NPs: BSA modified gadolinium (Gd)-integrated copper sulphide (CuS) nanotheranostic hybrid NPs; PCB1-Bro: a semiconducting polymer amphiphile (PCB) conjugated with bromelain (Bro); COF-909: a kind of covalent organic framework (COF); AuNC@DHLA: dihydrolipoic acid (DHLA)-coated gold nanocluster (AuNC); Zr-TBB: a nanoscale metal–organic framework (NMOF) based on 5,10,15,20-tetra (p-benzoato) bacteriochlorin (TBB) ligands; AIEgens: aggregationinduced emission (AIE) luminogens; TFPy/TFVP/TPE-TFPy: three AIEgens with the same backbone were tailored and used to specifically target mitochondria, cell membrane, and lysosome, respectively; AIE-PS@liposomes: AIE-photosensitizer (PS)-loaded liposome; mSiO2@PEI gene nanocarrier: upconverting rods (UCRs) conjugated with a mesoporous silica (mSiO2)@polyethyleneimine (PEI) nanocarrier; DOX: doxorubicin; AIPH: 2,2' -azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride; v-A-CED2 : modular nanovesicles containing oxidation-responsive nanovesicles (v), radical generators (A), and Edman linker conjugated prodrugs (CED2); CR780: croconaine dye; yolk–shell Fe3O4@Au NPs: yolk–shell structured magnetic–plasmonic hybrid NPs (MPHNs) comprising a ferroferric oxide (Fe3O4) core within a hollow cavity encircled by a porous Au outer shell; EPT1-GNR: a thiol-terminated and water-soluble 9,10-disubstituted anthracene endoperoxide derivative (EPT1) modified gold nanorod (GNR); LACP: lipidencapsulated, AuNPs-condensed Cas9-sgPlk-1 plasmids (CP); Ce6@PPE-TK-DOX NPs: DOX was conjugated to the side chain of polyphosphoesters (PPEs) by a thioketal (TK) bond (PPE-TK-DOX) and encapsulate the photosensitizer chlorin e6 (Ce6) during its self-assembly in aqueous solution; dNMs: decorated nanomedicines (NMs); ICG/TPZ@HSA dNMs: human serum albumin (HSA) NMs decorated with diazirine (DA) and loaded with ICG and tirapazamine (TPZ); R837@RBC-gel: red blood cells (RBC)-based hydrogel loading with immune adjuvant imiquimod (R837); ZCIS QD: CuInS/ZnS (ZCIS) quantum dots (QD); CNQD-CN-DOX: DOX-loaded graphitic carbon nitride QD (CNQD) embedded in carbon nanosheets (CN); PB@PEI/HSP70-p53-GFP: plasmid DNA (pDNA) (HSP70-p53-GFP)-loaded PEI-modified Prussian blue (PB) nanocubes (PB@PEI NCs); HPPH: 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-α; CPT: camptothecin; CPT-TK-HPPH/Pt NP: a platinum (Pt) nanozyme loaded reactive oxygen species (ROS)- responsive prodrug NP; TCPP: 4,4,4,4-(porphine-5,10,15,20-tetrayl) tetrakis(benzoic acid); NMOF@DHA@CaCO3: nanoplatform of dihydroartemisinin (DHA) with a ferrum (Fe)-TCPP NMOF having a calcium carbonate (CaCO3) mineralized coating.
《Fig. 1》
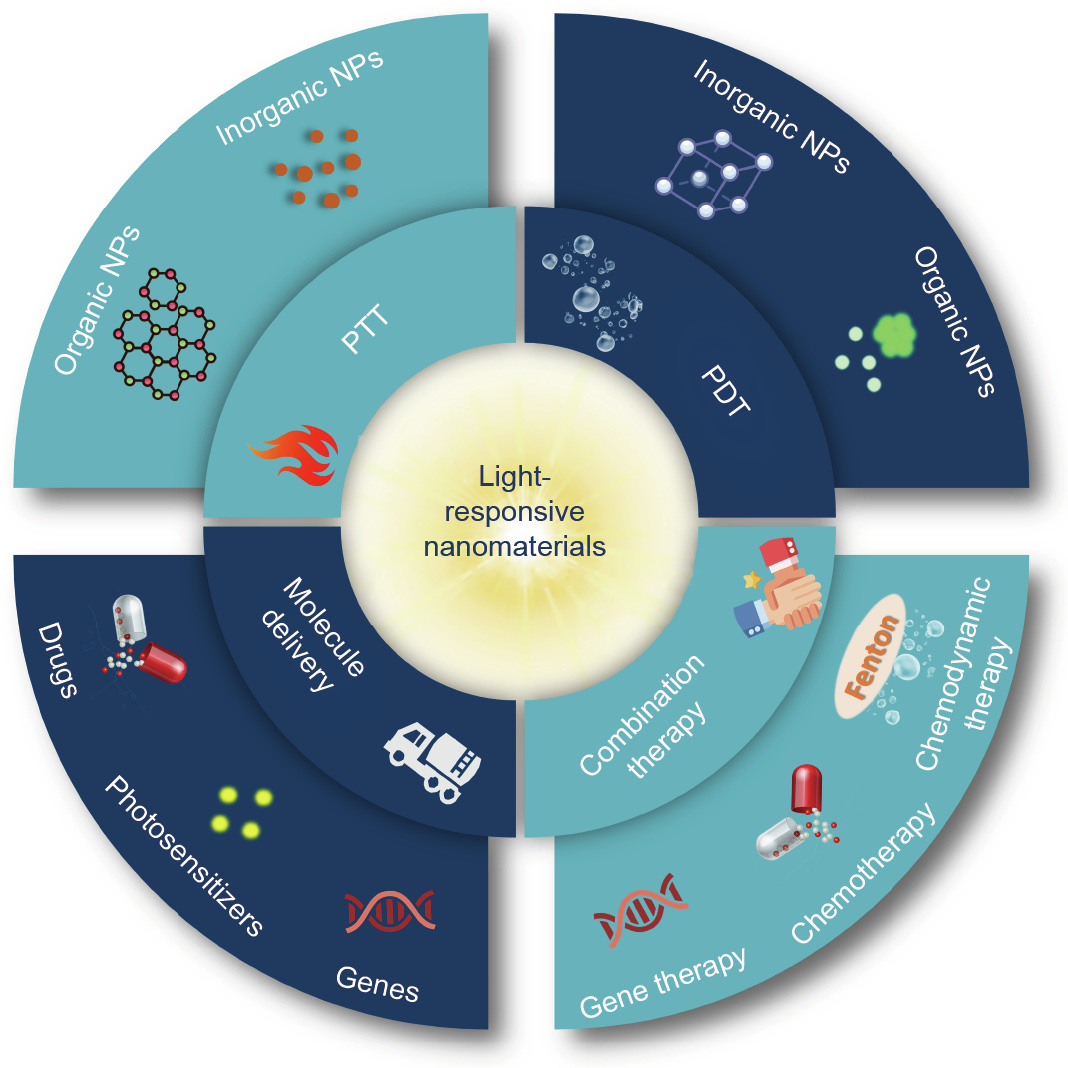
Fig. 1. Schematic illustration of applications of light-responsive nanomaterials in cancer therapy. NPs: nanoparticles.
《2. Light-responsive nanomaterials》
2. Light-responsive nanomaterials
Light-responsive nanomaterials that are used in cancer phototherapy include PTCAs, nanophotosensitizers, and nanoplatforms containing light-responsive moieties. PTCAs are agents that can capture light energy and convert it into heat to trigger cancer cell death. In general, PTCAs can be divided into two categories: organic materials and inorganic materials. Common organic PTCA materials include organic dye molecules (e.g., indocyanine green (ICG)) and organic nanoparticles (NPs) (e.g., semiconducting polymer NPs) [48,49]. Inorganic PTCA materials include noble metal materials (e.g., gold (Au) nanomaterials), transition metal chalcogenides and oxides (e.g., copper sulphide (CuS) NPs), and carbonbased materials (e.g., graphene oxide) [50].
Photosensitizers can be activated by light with a specific wavelength to generate reactive oxygen species (ROS) and damage cancer cells through local oxidative stress [51]. Photosensitizers can also be divided into organic materials and inorganic materials. Organic photosensitizers include organic dye molecules such as porphyrin and boron-dipyrromethene (BODIPY), while inorganic photosensitizers include titanium dioxide (TiO2), zinc oxide (ZnO), graphitic carbon nitride (g-C3N4), and more [52,53].
Many light-responsive nanoplatforms have been designed and developed by exploiting light-responsive linkers, such as azobenzene [54]. These nanoplatforms can be used as efficient nanocarriers to realize light-responsive molecule delivery. In addition to designing such nanoplatforms to directly respond to light, lightresponsive nanomaterials can be designed to respond via a cascade strategy [55]. For example, nanomaterials composed of photothermal agents (PTAs) and thermal-sensitive linkers can respond to light irradiation. Moreover, light-induced ROS generation can lead to the cleavage of some chemical bonds, such as thioketal (TK) linkers, thereby achieving light-triggered drug release [56].
《3. Photothermal therapy》
3. Photothermal therapy
PTT is a promising treatment with minimal invasiveness and high therapeutic effects in cancer therapy [57]. Under irradiation by external light, PTCAs absorb light energy and convert it into heat, which kills cancer cells. PTCAs usually need to have high NIR absorption and high photothermal conversion efficiency. In the past decades, various nanomaterial-based PTCAs, such as Au nanomaterials, black phosphorus (BP), niobium carbide, graphene, metallic/nonmetallic compounds, and organic dye, have been developed for PTT [23–26,58–60]. For example, El-Sayed et al. [61] reported that gold nanorod (GNR)-assisted plasmonic PTT is a potential and effective alternative to traditional surgery. Furthermore, GNR PTT can decrease the collective migration of cancer cells by regulating and remodeling actin filaments and cell junction proteins [62]. De Sio et al. [27] reported a keratin-coated biomimetic gold NP (Ker-AuNP) that can be used as a highly efficient PTCA for plasmonic PTT.
Zhang et al. [28] developed an inorganic nanotheranostic agent (Gd:CuS@bovine serum albumin (BSA) NPs) based on gadolinium (Gd)-integrated copper sulphide (CuS) NPs (Fig. 2 (a)). Due to the strong NIR absorbance of the Gd:CuS NPs [63– 65] and the good biocompatibility achieved by the BSA modification [66], the nanotheranostic agent (Gd:CuS@BSA NPs) can be used as an excellent nanotheranostic agent for in vivo photoacoustic (PA)/magnetic resonance imaging (MRI) guidance and effective PTT. As shown in Fig. 2(b), under NIR laser irradiation, the temperature of the Gd:CuS@BSA NPs clearly increased, indicating the good photothermal effect of Gd:CuS. Moreover, the Gd:CuS@BSA NPs exhibited the continuous enhancement of in vivo PA/MRI signals within 24 h on tumor-bearing mice (Fig. 2(c)). Dual-modal imaging facilitated imaging-guided phototherapy, resulting in potent tumor elimination. To verify the PTT effect of the Gd:CuS@BSA NPs in vitro, a cytotoxicity study and live/dead cell co-staining were carried out on SK-OV-3 cells. As shown in Figs. 2(d) and (e), the relative viabilities of the tumor cells were obviously decreased upon laser irradiation, which demonstrated the tumor-ablation effect of Gd:CuS@BSA. The photothermal effect of Gd:CuS@BSA was also demonstrated in vivo. As shown in real-time thermal images (Fig. 2(f)), when treated with Gd:CuS@BSA NPs, the temperature of the tumor sites increased by 21 °C, which was significantly higher than the temperature increase in the control group (6 °C). Under NIR laser irradiation, the tumor exhibited remarkable regression, followed by complete elimination (Fig. 2(g)), which demonstrated the significant tumor-ablation effect of PTT.
《Fig. 2》
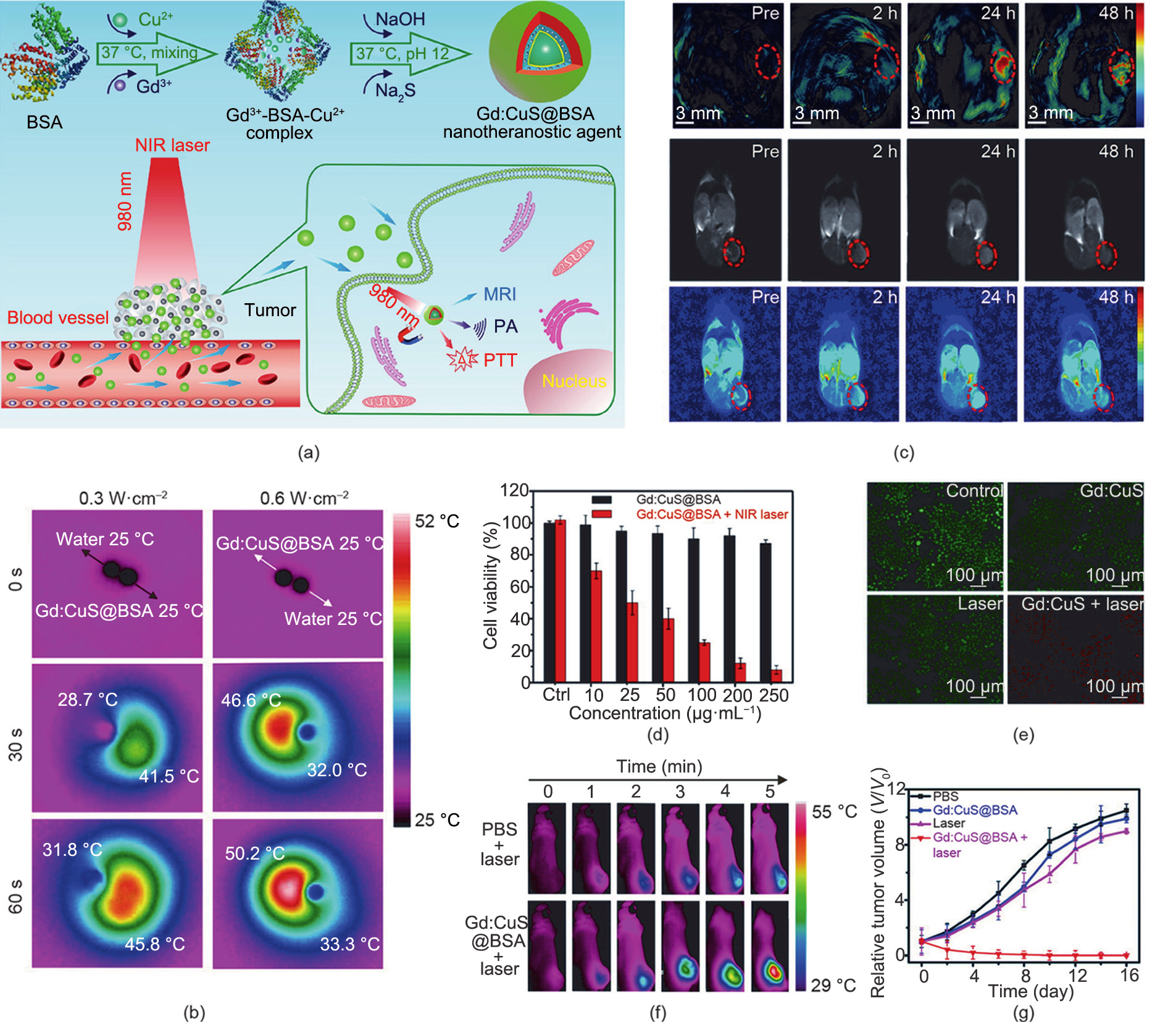
Fig. 2. (a) Schematic illustration of Gd:CuS@BSA hybrid theranostic agents for imaging-guided tumor PTT. MRI: magnetic resonance imaging. (b) Infrared thermal images of an aqueous Gd:CuS@BSA NPs droplet of a Gd:CuS@BSA NP and a deionized water droplet with NIR laser irradiation (980 nm). (c) PA/MRI (dual-modal imaging) of an SK-OV-3 tumor before and after Gd:CuS@BSA treatment. (d) Viability of SK-OV-3 cells treated with Gd:CuS@BSA NPs and laser irradiation (980 nm, 0.6 W∙cm–2 , 5 min). Ctrl: control group (treated with 0 μg∙mL–1 Gd:CuS@BSA NPs). (e) Fluorescence staining of live/dead SK-OV-3 cells after treatment with different formulations. (f) In vivo thermal imaging of an SK-OV-3 tumor treated with different formulations followed by laser irradiation (980 nm, 5 min). (g) Growth curves of an SK-OV-3 tumor treated with different formulations. Reproduced from Ref. [28] with permission of American Chemical Society, © 2016.
Apart from inorganic nanomaterials, organic nanoagents play essential roles in cancer PTT. For example, US Food and Drug Administration (FDA)-approved ICG, a highly biocompatible NIR organic dye, has been widely applied in PTT due to its various energy level transition pathways upon NIR light excitation [67,68]. In addition, Pu et al. [29] reported semiconducting polymer nanoenzymes (SPNs) with an NIR photothermic effect for enhanced cancer therapy. As shown in Fig. 3(a), the SPNs contain a semiconducting polymer amphiphile (PCB) as a photothermal nanotransducer and bromelain (Bro) as a temperature-sensitive enzyme. Under NIR laser irradiation, the local temperature increased due to the photothermal conversion activity of the SPNs, leading to the photothermic activation of Bro. Therefore, collagen, the most abundant protein in the tumor extracellular matrix [69], was digested in situ, resulting in the improved tumor accumulation of SPNs and enhanced PTT. Upon 808 nm laser irradiation, the temperatures of the PCB1-Bro group were obviously increased, both in vitro (Fig. 3(b)) and in vivo (Fig. 3(c)), which verified the high photothermal conversion efficiency of PCB1-Bro. Moreover, the penetration depth of PCB1-Bro with NIR laser irradiation was evidently enhanced in comparison with the penetration depth without laser irradiation, confirming that photothermal-enhanced collagen digestion had occurred. As shown in Fig. 3(d), after the photothermic activation of the Bro enzyme, the tumor fluorescence signals of the PCB1-Bro-treated group were higher than those of other groups, indicating enhanced tumor accumulation of PCB1-Bro due to collagen digestion. The efficient accumulation of PCB1-Bro further led to a higher tumor temperature and stronger tumor growth inhibition (Figs. 3(c) and (e)).
Due to the advantages of PTT, some PTCAs have already entered clinical trials. In 2019, the results of a phase I trial demonstrated the feasibility of an approach involving gold–silica nanoshells for the focal PTT ablation of prostate tumors [70]. In addition, in order to further improve the light penetration depth, NIR-II light responsive PTCAs have gained increased research attention. For example, Yang et al. [71] developed a polyethylene glycol-graftpolyethylenimine-modified hollow carbon nanosphere for NIR-II laser-activated cancer PTT.
《Fig. 3》
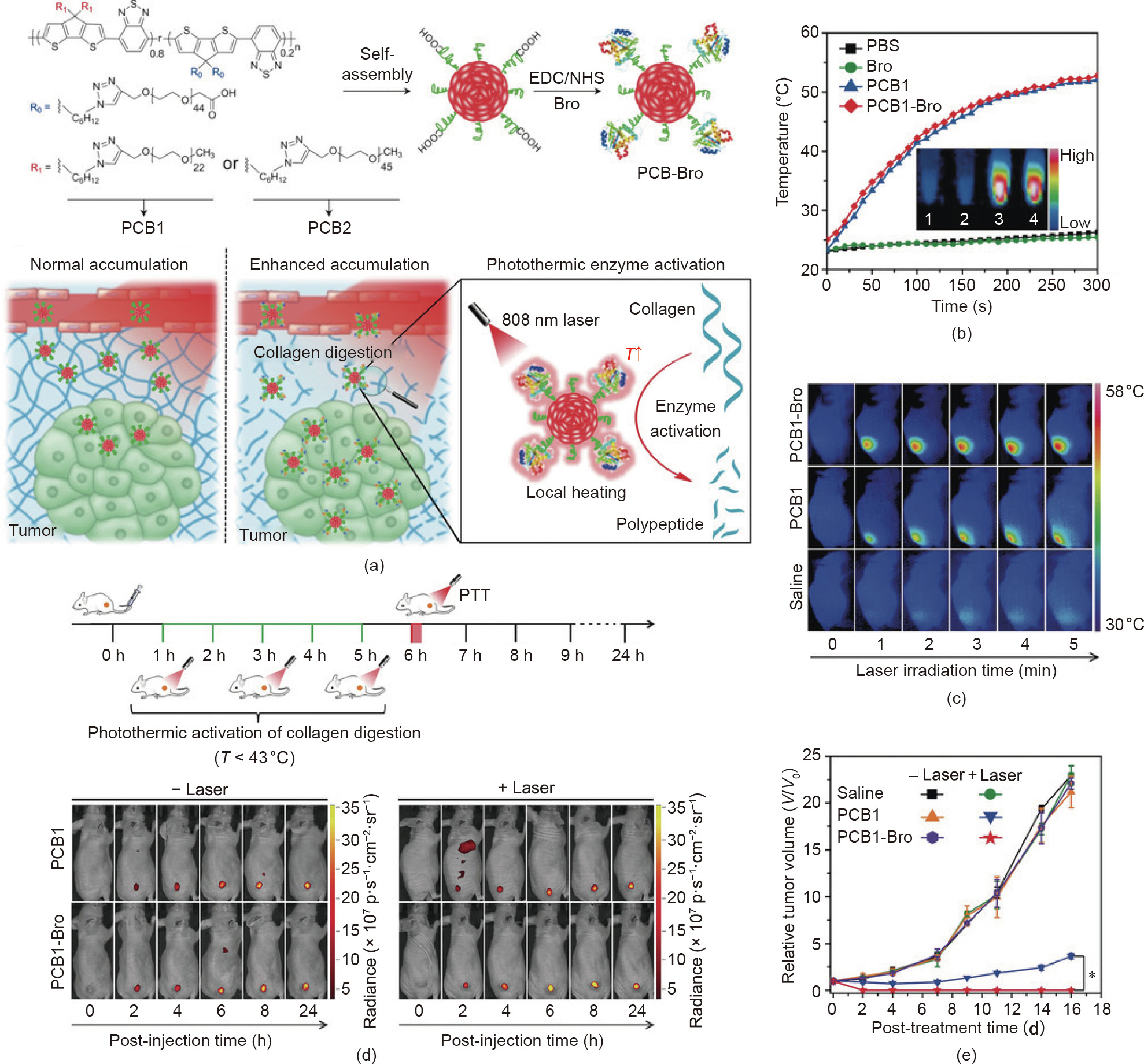
Fig. 3. (a) Schematic illustration of PCB1-Bro for photothermally enhanced NP accumulation in tumors. EDC/NHS: 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/Nhydroxysuccinimide. (b) Temperature changes of different samples upon laser irradiation. (c) Infrared thermal images of 4T1 tumor-bearing mice treated with different formulations and laser irradiation (808 nm, 5 min). (d) Real-time fluorescence images of 4T1 tumor-bearing mice treated with different formulations with/without laser irradiation. (e) Growth curves of a 4T1 tumor with different treatments. PBS: phosphate buffered saline. Reproduced from Ref. [29] with permission of Wiley-VCH Verlag GmbH & Co. KGaA, © 2018.
《4. Photodynamic therapy》
4. Photodynamic therapy
PDT is a clinically approved modality that has been used for more than 40 years in the treatment of cancers, including superficial skin lesions, esophageal tumors, and lung tumors [48]. There are three essential elements in PDT: oxygen, a photosensitizer, and light [72]. Hundreds of photosensitizers have been applied for PDT clinically or preclinically, including porphyrin, chlorin, and phthalocyanine derivatives [73,74]. In a conventional PDT process, photosensitizers transfer light energy to surrounding molecules to generate cytotoxic ROS for cancer treatment [75–77]. There are two main types of photodynamic reaction—type I and type II. In the mechanism of a type I reaction, a triplet-state photosensitizer reacts with cellular substrates directly via electron transfer, resulting in the generation of free radicals. The generated radicals then react with oxygen (O2) to produce oxygenated products, including superoxide anions (O2– ), hydrogen peroxide (H2O2), and hydroxyl radicals (· OH). Alternatively, in the mechanism of a type II reaction, a triplet photosensitizer transfers energy directly to oxygen (3O2) to produce highly reactive singlet oxygen (1O2)[14,73,76]. Most of the existing PDT systems adopt the oxygen dependent type II mechanism [78].
Compared with other conventional therapeutic methods, PDT possesses several advantages, including minimal invasiveness, the spatial accuracy of the operation, minimal side effects, non-drug resistance, and a short recovery period [79]. However, PDT also has major restrictions, including the hypoxic condition of the tumor, the low targeting efficiency of the photosensitizer, and limited light penetration depth. In addition, the nonspecific distribution of photosensitizers causes phototoxicity in normal tissues, which must be taken into consideration. Due to stringent requirements regarding biocompatibility and the frontier orbitals of the molecular motifs, the choice of efficient molecular photosensitizers remains limited [30]. To address these problems, many novel photosensitizers with versatile properties have been developed to enhance the efficacy of PDT. For example, porous covalent organic frameworks (COFs) with excellent ROS production efficiency have been developed by linking ROS-inert molecular motifs [30]. To enhance the effects of PDT in deep tissues, a combination of UCNPs and photosensitizers is an efficient approach [80]. Xing et al. [81] proposed a strategy to achieve highly efficacious PDT by integrating UCNPs with graphene quantum dots (GQDs). UCNPs can serve as light transducers to convert NIR light into visible light for photosensitizer activation, thus greatly expanding the applicability of PDT.
Moreover, some studies have demonstrated that the radicals generated via a type I photoreaction can amplify PDT response, especially under hypoxic conditions [82]. Based on this, Yoon et al. [83] reported a nanostructured phthalocyanine assembly (NanoPcA), which has a favorable antibacterial effect through enhanced type I PDT. Jiang et al. [31] developed a type I nanoagent—namely, dihydrolipoic-acid-coated gold nanoclusters (AuNC@DHLA)—for two-photon anticancer PDT. In this nanoagent, Au nanoclusters are used as a promising photosensitizer due to their excellent biocompatibility and long fluorescence tripletstate lifetime [84]. In another study, Lin et al. [32] reported a nanoscale metal–organic framework (NMOF) based on 5,10,15,20-tetra (p-benzoato) bacteriochlorin (TBB) ligands (denoted as Zr-TBB) for enhanced PDT via both type I and type II mechanisms (Fig. 4(a)). As a nanophotosensitizer, NMOF possesses the unique properties of high photosensitizer loading efficacy, a porous structure, and intrinsic biodegradability [85]. Bacteriochlorin exhibits excellent absorption in the NIR region (700–850 nm) and can be used for type I PDT under hypoxic conditions [86]. However, the PDT efficacy of bacteriochlorin is significantly restricted by its instability to oxygen and light [87,88]. The structure of NMOF is beneficial for stabilizing bacteriochlorin’s ligands, thereby preventing unimolecular photodecomposition and selfquenching. As shown in the confocal laser scanning microscopy (CLSM) images in Fig. 4(b), four types of ROS (O2– , H2O2, ·OH, and 1O2) were produced in the Zr-TBB group, confirming the presence of light-induced type I and type II PDT processes. Accordingly, Zr-TBB exhibits excellent in vivo antitumor efficacy against both subcutaneous 4T1 and MC38 tumors (Fig. 4(c)).
《Fig. 4》

Fig. 4. (a) Schematic illustration of bacteriochlorin ligands stabilized in Zr-TBB for type I and type II PDT. (b) CLSM images of ROS generation in 4T1 cells treated with different formulations after light irradiation. Scale bars: 20 lm. (c) Growth curves of a 4T1 tumor and an MC38 tumor treated with different formulations. Reproduced from Ref. [32] with permission of American Chemical Society, © 2020.
Traditional photosensitizers, such as porphyrin derivatives, are hydrophobic and tend to aggregate in aqueous solution. The aggregation state of photosensitizers causes fluorescence quenching, which reduces the ROS production efficiency and greatly limits the PDT treatment outcome [89,90]. To address this issue, Chen et al. [91] developed a single molecular nanophotosensitizer based on a porphyrin nanocage. Due to the rigid structure of the nanocages, the π–π stacking interaction between the photosensitizers is inhibited, which promotes the photosensitizing effect.
The development of aggregation-induced emission (AIE) photosensitizers provides another strategy to overcome the quenching effect of traditional photosensitizers [92,93]. Recently, Tang et al. [33] proposed a ‘‘1 + 1 + 1 > 3” synergistic strategy based on three AIE luminogens (AIEgens). The AIEgens have the same photosensitizer backbone but different targeting groups that can target mitochondria, the cell membrane, and lysosomes, respectively (Fig. 5(a)). As shown in Fig. 5(b), after cellular uptake by 4T1 cells, the three AIEgens manifested excellent co-localization with corresponding organelles. The tumor-inhibition effect of this three-inone group was found to be better than that of each photosensitizer alone, which was ascribed to the synergistic PDT strategy (Fig. 5(c)).
《Fig. 5》
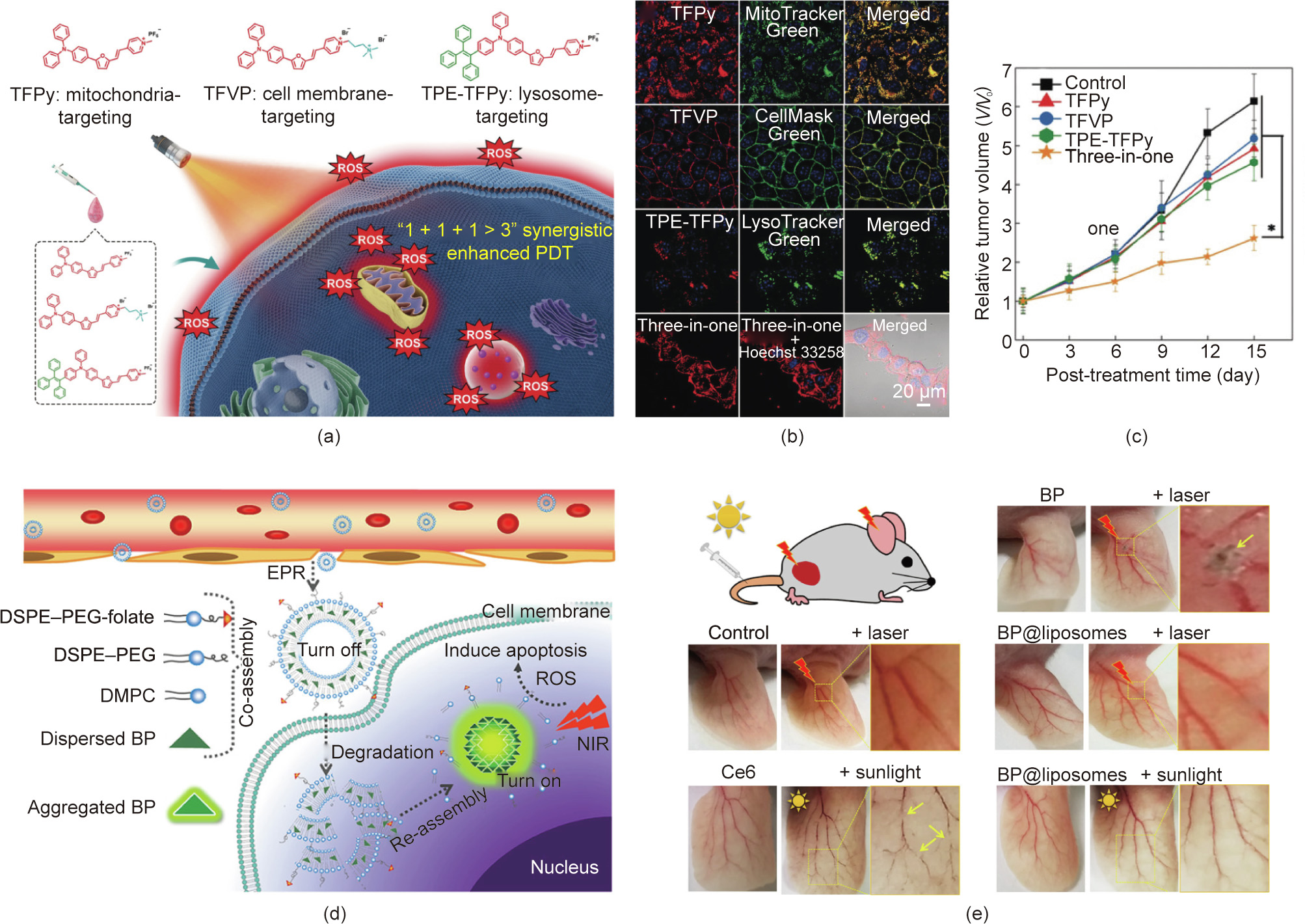
Fig. 5. (a) Chemical structures and schematic illustration of three AIEgens for synergistically enhanced PDT. (b) Co-localization of the AIEgens in CLSM images of 4T1 cells treated with different formulations. (c) Growth curves of a 4T1 tumor treated with different formulations. (d) Schematic illustration of AIE-PS@liposomes for activatable PDT. DSPE-PEG: distearoylphosphatidylethanolamine-poly(ethylene glycol) 2000; DMPC: 1,2-dimyristoyl-sn-glycero-3-phosphocholine. (e) Blood vessels in ears treated with different formulations. (a)–(c) Reproduced from Ref. [33] with permission of Wiley-VCH Verlag GmbH & Co. KGaA, © 2020; (d) and (e) reproduced from Ref. [34] with permission of American Chemical Society, © 2019.
Due to the nonspecific biodistribution of photosensitizers, most photosensitizers will trigger phototoxicity when patients are exposed to sunlight. To reduce the phototoxicity, activatable PDT is a promising strategy. For example, Li et al. [34] developed an AIE-photosensitizer-loaded liposome (AIE-PS@liposome) to achieve controlled photosensitization. As shown in Fig. 5(d), the photosensitivity of the AIE-PS entrapped in the lipid bilayer is in an ‘‘OFF” state; however, once the AIE-PS@liposomes reach tumor sites, the AIE-PS are released for in situ reaggregation, leading to activated PDT. As expected, the AIE-PS@liposomes exhibited much lower phototoxicity under both laser and sunlight irradiation in comparison with the control groups, which included AIE-PS nanoaggregates and the commercial photosensitizer chlorin e6 (Ce6) (Fig. 5(e)).
《5. Light-responsive molecule delivery》
5. Light-responsive molecule delivery
The low targeting of therapeutic agents in cancer therapy results in unsatisfactory efficacy and severe side effects in normal tissues. With the development of stimuli-responsive delivery systems, an increasing number of nanocarriers based on polymeric and inorganic nanomaterials have been exploited to realize the targeted delivery of drugs, photosensitizers, genes, and so forth [94– 98]. As an external stimulus, light has unique features for controlling the release behaviors of therapeutic agents at precise locations and avoiding individual variability [35,99]. For example, thermalresponsive nanomaterials can achieve light-triggered drug delivery via photothermal-induced linker cleavage, structural changes in nanocarriers, radical generation, and other pathways [36–39]. The ROS produced by photosensitizers under light irradiation can also lead to on-demand drug delivery by triggering the cleavage of ROS-sensitive linkers, such as TK linker [40,100].
Liang et al. [41] developed diazirine (DA)-decorated human serum albumin (HSA) nanomedicines (denoted as HSA dNMs) for the co-encapsulation of photosensitizer (ICG) and the prodrug tirapazamine (TPZ) via electrostatic and hydrophobic interactions (Fig. 6(a)). As shown in Fig. 6(b), under 405 nm laser irradiation, the DA groups transformed into reactive carbine moieties and reacted with the adjacent HSA dNMs for crosslinking, leading to improved tumor accumulation and retention of ICG/TPZ@HSA dNMs. Next, 808 nm laser irradiation triggered hyperthermia and ROS generation for tumor PTT and PDT. In addition, the oxygen consumption in the PDT process led to hypoxia aggravation and TPZ activation to achieve synergistic therapy. The lightresponsive delivery of HSA dNMs was confirmed by both in vitro and in vivo results. The size of the HSA dNMs with DA modification was clearly increased after exposure to a laser at 405 nm (Fig. 6(c)), which verified the UV-light-triggered aggregation behavior. The tumor accumulation of the ICG/TPZ@HSA dNMs in vivo was represented by the imaging contrast index (CI) calculated from the fluorescent signal ratio of tumor to tissue [101]. The CI values of the UV-laser-treated group were significantly higher than those of the control group without laser irradiation, confirming the laser enhanced tumor accumulation behavior of the ICG/TPZ@HSA dNMs (Fig. 6(d)). Consistent results were obtained, based on biodistribution fluorescence images. As shown in Fig. 6(e), the fluorescence intensity of the tumors was significantly increased after laser treatment. This elaborate design of ICG/TPZ@HSA dNMs enhanced the targeted delivery of ICG/TPZ and improved the anticancer efficacy through light-induced aggregation and cascade phototherapy/ chemotherapy.
《Fig. 6》
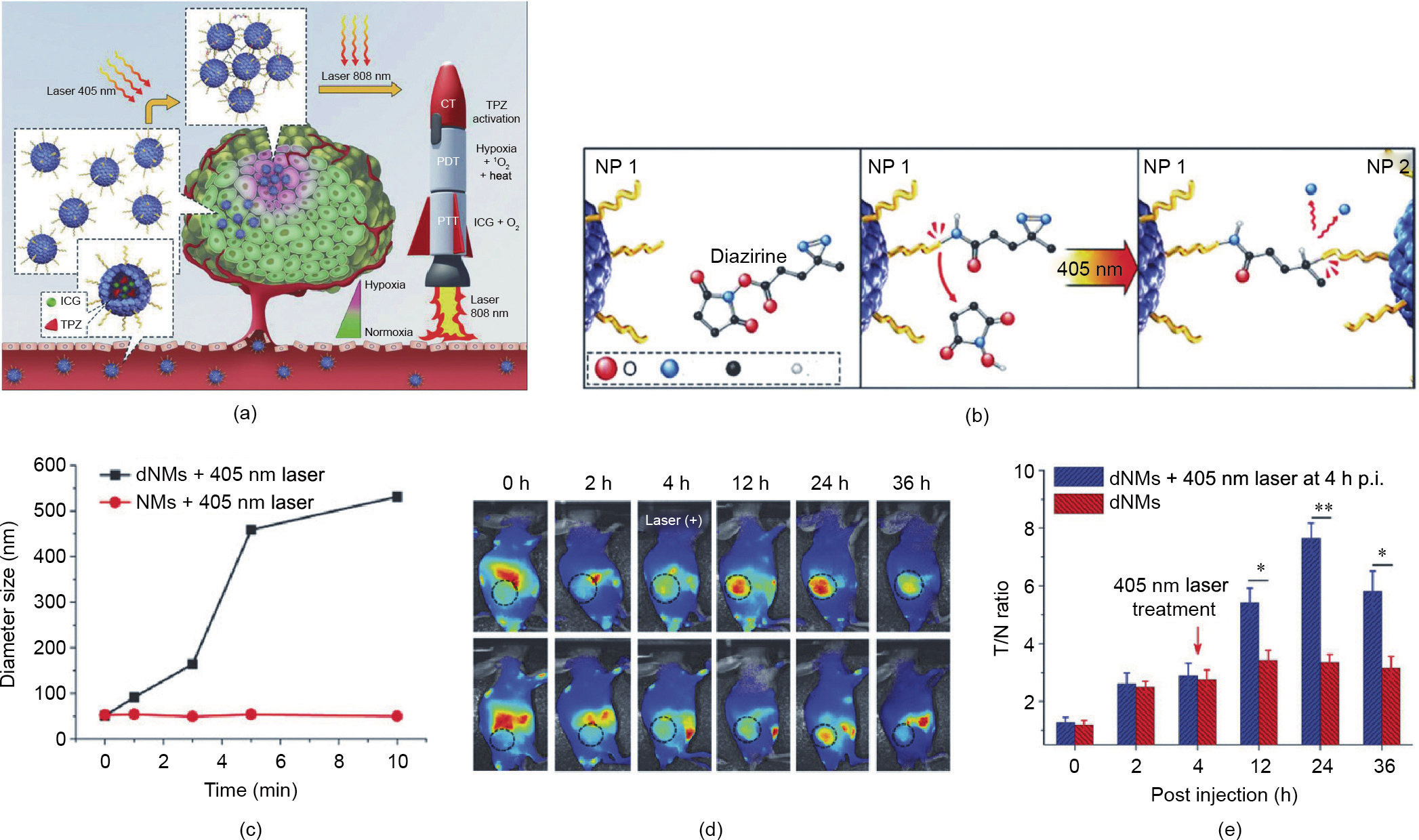
Fig. 6. Light-responsive drugs/photosensitizers delivery system for cancer therapy. (a) Schematic illustration of ICG/TPZ@HSA dNMs for cascaded synergistic cancer therapy triggered by laser irradiations (405 and 808 nm). CT: chemotherapy. (b) Scheme of 405 nm laser-induced aggregation of ICG/TPZ@HSA dNMs. (c) Size changes of HSA dNMs with different sequential laser irradiation times. (d) Tumor accumulation of ICG/TPZ@HSA dNMs, as represented by CI values. (e) In vivo biodistribution of ICG/TPZ@HSA dNMs in 4T1 tumor-bearing mice. T/N: the fluorescence signal ratio of tumor to tissue; p.i.: postinjection. Reproduced from Ref. [41] with permission of Wiley-VCH Verlag GmbH & Co. KGaA, © 2018.
Aside from the delivery of drugs and photosensitizers, nanomaterials can be applied for the light-controlled selective activation of gene expression in gene therapy. For example, optogenetics is a combination of optical and genetic methods to precisely control the expression of proteins in specific cells, so as to control intracellular biological processes and behaviors [102]. Like most lighttriggered delivery systems that rely on UV/visible light irradiation, the activation of the photo-actuator in optogenetics is seriously restricted by the limited light penetration depth [103–105]. To address this issue, UCNPs can serve as a nanotransducer to transform external NIR light with deep penetration depth into local UV/visible light for the noninvasive activation of photoreceptors in vivo [106,107].
Chang et al. [108] developed an upconversion optogenetic nanosystem composed of UCNPs, arabidopsis flavoprotein cryptochrome 2 (Cry2), and its interacting partner Cib1 plasmids. The UCNPs play a part in both plasmid DNA (pDNA) delivery and light transformation. External NIR-light-triggered local blue light emitting noninvasively induces photoreceptor Cry2 and Cib1 interaction, which activates the apoptotic signaling pathway of cancer cells. In another effort, Chang’s group [35] reported on an NIRcontrolled gene-delivery system based on mesoporous silica (mSiO2)@polyethyleneimine (PEI) nanocarrier-conjugated upconverting rods (UCRs) (Fig. 7(a)). Upon NIR light irradiation, the external NIR light is converted into UV light by the UCRs to trigger the cleavage of o-nitrobenzyl groups (Fig. 7(b)). As a result, the gene-loaded mSiO2@PEI nanocarriers are released for gene delivery (Fig. 7(c)). This gene expression study reported that the genes were released and expressed upon NIR irradiation, confirming that lightcontrolled precisely regulated gene expression at high spatial and temporal resolutions had been achieved.
《Fig. 7》
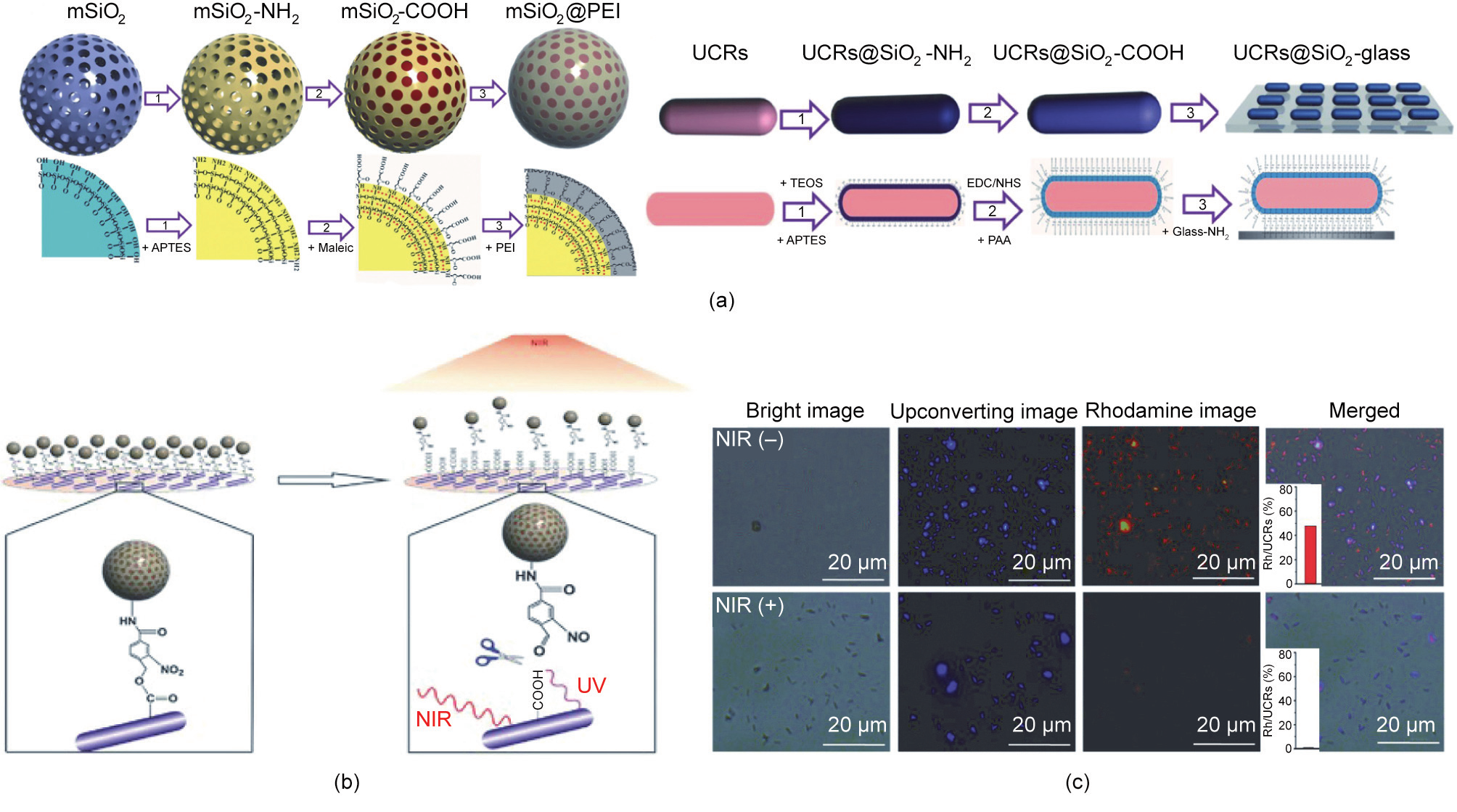
Fig. 7. Light-responsive gene delivery and expression. (a) Synthetic procedure for the mSiO2@PEI gene nanocarrier and the UCR substrate. APTES: 3-aminopropyl triethoxysilane; TEOS: tetraethyl orthosilicate; PAA: poly(acrylic acid). (b) Schematic illustration of the light-triggered release of mSiO2@PEI gene nanocarriers from the UCR substrate due to photocleavage of o-nitrobenzyl groups. (c) Fluorescence images of the substrate immobilized with rhodamine-loaded mSiO2@PEI nanocarriers with or without NIR laser (980 nm, 8.9 W∙cm–2 , 12 min). Reproduced from Ref. [35] with permission from Wiley-VCH Verlag GmbH & Co. KGaA, © 2015.
《6. Light-controlled combination therapy》
6. Light-controlled combination therapy
Although phototherapy techniques have been developed in recent years, some drawbacks of single-mode phototherapy, which relies on only one therapeutic method, remain to be resolved, such as relapse or metastasis in the treatment of persistent tumors [1]. To address this issue, combination phototherapy with immunotherapy, gene therapy, chemotherapy, chemodynamic therapy (CDT), and other therapy methods can maximize the advantages of each therapeutic pattern and achieve complementary multitherapeutic efficacy [109,110].
Photo-immunotherapy, which can eliminate primary tumors and induce host immunity in order to control distant metastases, is considered to be a promising strategy for the treatment of metastatic cancer [111]. For example, Wang et al. [42] developed an injectable red blood cell (RBC)-based gel for cancer photoimmunotherapy. After the subcutaneous injection of imiquimod (R837) adjuvant engineered RBCs, a hydrogel-like composition spontaneously formed due to the infiltrated platelets and thrombin. The photothermal effect of the in situ-formed RBC gel led to the photoablation of tumors and the production of tumorassociated antigens, thereby initiating adaptive immune responses to cancer. Moreover, the released immunoadjuvant, R837, induced robust and persistent immune responses for cancer metastasis/ recurrence inhibition.
Liang et al. [43] developed an ‘‘all-in-one” theranostic nanomedicine based on CuInS/ZnS (ZCIS) quantum dots (QDs) for synergistic PTT/PDT therapy. QDs have been widely used in tumor diagnosis and treatment due to their high fluorescence intensity and broad excitation spectrum [44,112,113]. The efficient absorption effect of ZCIS QDs in the range of 650–750 nm has enabled noninvasive fluorescence/multispectral optical tomography imaging. Furthermore, the photothermal and photodynamic effects of ZCIS QDs could be used for cancer treatment (Fig. 8(a)).
In addition, Chang et al. [45] reported a multifunctional nanotheranostic agent (PB@PEI/HSP70-p53-GFP NP) based on human HSP70 promoter-loaded Prussian blue (PB) nanocubes for NIR light-triggered PTT/gene combination therapy. As shown in Fig. 8(b), due to the photothermal property of the PB nanocubes, the HSP70 promoter was activated under mild NIR laser irradiation ( ≈41 °C), resulting in tumor suppressor p53-dependent apoptosis. Under strong NIR laser irradiation ( ≈50 °C), both PTT and gene therapy were activated. Thus, the synergistic antitumor efficacy can be adjusted by the NIR laser irradiation.
The combination of chemotherapy and phototherapy is another important approach to realize synergistic therapy. Qian et al. [46] developed an ROS-responsive prodrug delivery nanoplatform for a combination of chemotherapy and PDT. As shown in Fig. 8(c), the chemotherapeutic drug camptothecin (CPT) and the photosensitizer pyropheophorbide-a (PPa) were conjugated to methoxypolyethylene glycol (MPEG) to prepare a polyprodrug, which further self-assembled into nanomedicine. The fluorescent PPa precisely located the tumor site and guided laser irradiation. Next, the ROS generated from the PPa-mediated PDT process broke the ROS-responsive TK linker between the polymer and CPT, leading to controlled release of the CPT drugs. This combination of chemotherapy and PDT exhibited a more effective antitumor effect than monotherapy.
Similar to PDT, CDT involves the generation of highly oxidative hydroxyl radicals for tumor treatment [114–117]. Tang et al. [47] constructed a programmed release system (NMOF@dihydroartemisinin (DHA)@calcium carbonate (CaCO3)) based on DHA-loaded ferrum (Fe)-(4,4,4,4-(porphine-5,10,15,20-tetrayl) tetrakis(benzoic acid)) (TCPP) NMOF with CaCO3 mineralized coating (Fig. 8(d)). The weak acidity of the tumor microenvironment led to the dissolution of the CaCO3 layer, resulting in Ca2+ release. Once the NMOF@DHA entered cancer cells, the high glutathione (GSH) level inside the cells led to Fe3+ reduction and TCPP activation, due to NMOF skeleton collapse. Therefore, NMOF@DHA@CaCO3 was able to realize Ca2+-DHA-mediated oncosis therapy, Fe2+- DHA-mediated CDT, and TCPP-mediated PDT.
《Fig. 8》
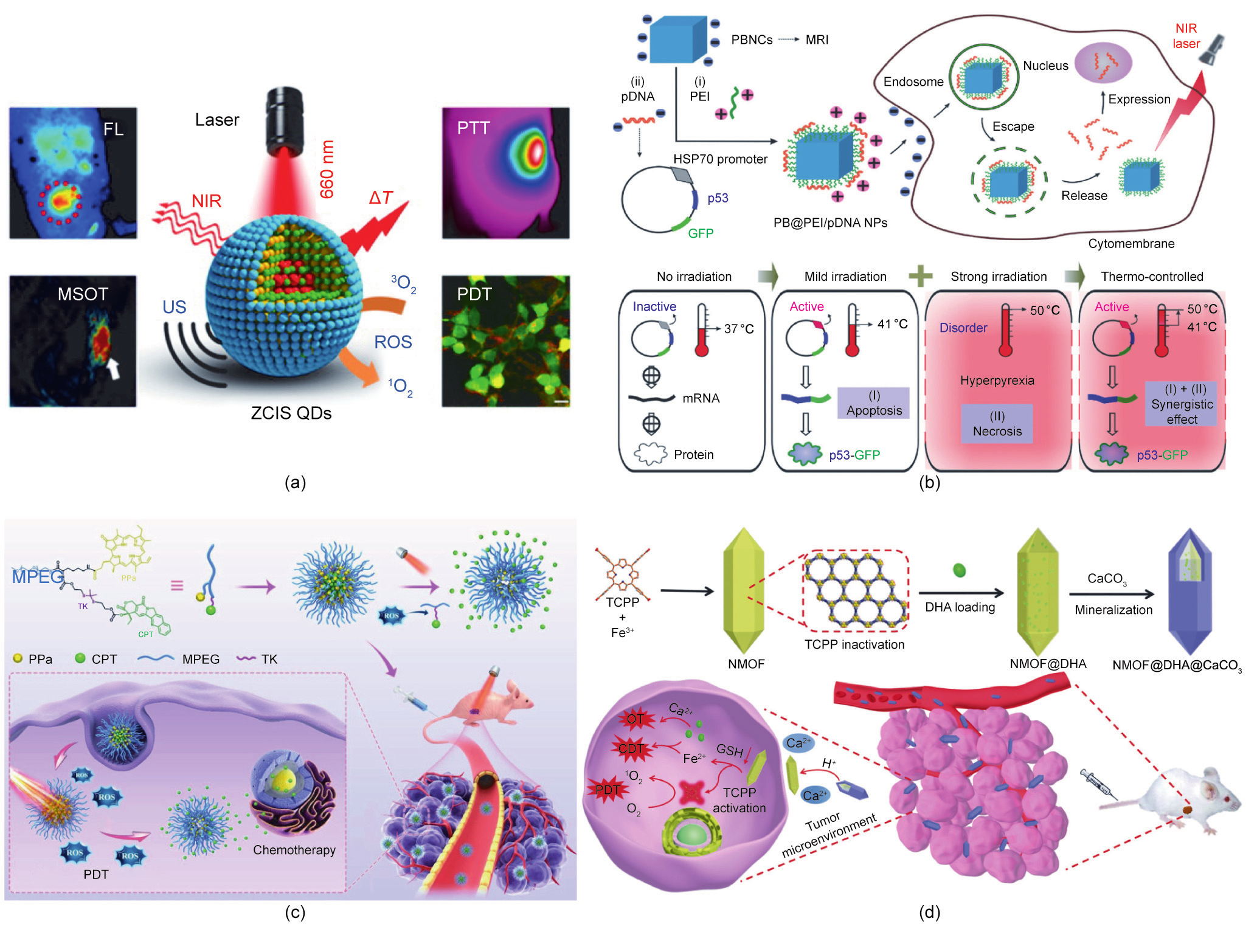
Fig. 8. Light-controlled combination cancer therapy. (a) Schematic illustration of ZCIS QDs in the combination therapy of PTT and PDT. FL: fluorescence; MSOT: multispectral optoacoustic tomography; US: ultrasound;  T: temperature increase. (b) Schematic illustration of PB@PEI/HSP70-p53-GFP NPs in the combination therapy of gene therapy and PDT. (c) Schematic illustration of MPEG–(TK–CPT)–PPa in the combination therapy of chemotherapy and PDT. (d) Schematic illustration of NMOF@DHA@CaCO3 in the combination therapy of CDT and PDT. (a) Reproduced from Ref. [43] with permission of American Chemical Society, © 2016; (b) reproduced from Ref. [45] with permission of Wiley-VCH Verlag GmbH & Co. KGaA, © 2018; (c) reproduced from Ref. [46] with permission of Wiley-VCH Verlag GmbH & Co. KGaA, © 2020; (d) reproduced from Ref. [47] with permission from Wiley-VCH Verlag GmbH & Co. KGaA, © 2019.
T: temperature increase. (b) Schematic illustration of PB@PEI/HSP70-p53-GFP NPs in the combination therapy of gene therapy and PDT. (c) Schematic illustration of MPEG–(TK–CPT)–PPa in the combination therapy of chemotherapy and PDT. (d) Schematic illustration of NMOF@DHA@CaCO3 in the combination therapy of CDT and PDT. (a) Reproduced from Ref. [43] with permission of American Chemical Society, © 2016; (b) reproduced from Ref. [45] with permission of Wiley-VCH Verlag GmbH & Co. KGaA, © 2018; (c) reproduced from Ref. [46] with permission of Wiley-VCH Verlag GmbH & Co. KGaA, © 2020; (d) reproduced from Ref. [47] with permission from Wiley-VCH Verlag GmbH & Co. KGaA, © 2019.
《7. Conclusions and future perspectives》
7. Conclusions and future perspectives
Light-induced phototherapy has been widely used to combat cancers due to its minimal invasiveness and minor side effects [118]. However, the efficacy of phototherapy is restricted by the limited depth of light penetration and the untargeted distribution of phototherapeutic agents. The emergence of light-responsive nanomaterials provides a promising approach to address these issues due to the specific properties of such nanomaterials, which include their nanoscale size, multifunctional surface modifications, and controllable synthesis. As a result, enhanced phototherapy has been achieved through the rational design of light-responsive nanomaterials.
This review summarized recent advances in light-responsive nanomaterials for cancer therapy, including PTT, PDT, lightresponsive molecule delivery, and light-controlled combination therapy. To achieve precise cancer phototherapy and accelerate clinical transformation, the following aspects should be taken into consideration:
(1) Limitation of light penetration depth. The penetration depth of light relies on the light wavelength. UV light and visible light are mainly applied in the treatment of superficial skin diseases due to their limited penetration depth. In addition, light is attenuated to a large extent when it interacts with tissues, which hinders the applications of phototherapy, especially in the treatment of deep-tissue tumors. Although the application of UCNPs provides an approach to increase light penetration depth, UCNPs still have the disadvantage of low conversion efficiency. Compared with the NIR-I window (750–1000 nm), the NIR-II window (1000– 1350 nm) exhibits reduced light scattering, minimized tissue absorption, and higher maximum permissible laser exposure; thus, it seems to be a prospective candidate for achieving deep-tissue penetration depth [119,120]. Therefore, NIR-II-responsive nanomaterials show great potential for expanding the application of phototherapy.
(2) Risk of potential toxicity. In the light-triggered molecule delivery process of phototherapy, the uncontrolled burst release of drugs from nanocarriers leads to short-term toxicity, while slow and incomplete metabolism of nanomaterials may result in longterm toxicity [121]. Furthermore, the untargeted biological distribution of light-responsive nanotherapeutic agents may cause sunlight-exposure-induced systemic toxicity. In order to realize precise controllable cancer phototherapy, an ideal nanomedicine should only be activated at the tumor site, and should maintain an ‘‘OFF” state in normal tissues. Therefore, the development of activatable phototherapeutic agents that can respond to the typical characteristics of the tumor microenvironment is an effective way to address the issue of phototoxicity.
(3) Clinical transformation. In order to achieve multifunctional cancer therapy, nanomaterials are usually complex in both design and composition, which undoubtedly introduces difficulties into their clinical transformation. In addition, due to the unknown toxicity mechanism of some newly developed nanomaterials, the clinical application of these nanomaterials requires complex toxicity-assessment procedures.
《Acknowledgments》
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32000991 and 51873150), the Young Elite Scientists Sponsorship Program by Tianjin (TJSQNTJ-2020-02), the Key Project of Tianjin Foundational Research (Jing–Jin–Ji) Program (19JCZDJC64100), and the Tianjin Research Innovation Project for Postgraduate Students (2020YJSB130).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Xu Zhang, Sheng Wang, Guohui Cheng, Peng Yu, and Jin Chang declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




