Glucose and lipids, along with amino acids, are the main energy sources of cells. Lipids also contribute to signaling processes and the maintenance of cell membrane integrity. Abnormal glucose and lipid metabolisms lead to diabetes, hyperlipidemia, obesity, and hypertension, which are collectively known as metabolic syndrome. With increasing nutritional disparities related to food supply and security, socio-economic factors, and rapid changes in our lifestyle, the cardiovascular complications of metabolic syndrome now present themselves as one of the major public health challenges facing the world today. In addition to well-known factors such as insulin, the unknown and unconventional pathogenic factors in metabolic syndrome must be exposed. There is increasing demand for an indepth exploration of the molecular mechanisms and novel therapeutic strategies for metabolic syndrome and its cardiovascular complications. To this end, leading researchers were invited to share their findings and views on current topics involving the regulation of glucose and lipid metabolism and cardiovascular health, and these are presented in this Special Issue as a collection of original research reports and review articles. The goal of this Special Issue is to provide the most updated and comprehensive discoveries and opinions to help in deciphering the complexity of metabolic syndrome and to pave the way for novel ideas and improved strategies for battling related cardiovascular complications.
The development of obesity has multiple etiologies. As reported in this issue, Verma et al. find that hydrogen sulfide (H2S), which is generated through cystathionine 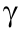 -lyase (CSE), is a pathogenic factor in obesity—in direct contrast to the cardiovascular protective role of this gasotransmitter. It is known that H2S stimulates the adipogenesis of 3T3L1 cells through the S-sulfhydration of peroxisome proliferator-activated receptor
-lyase (CSE), is a pathogenic factor in obesity—in direct contrast to the cardiovascular protective role of this gasotransmitter. It is known that H2S stimulates the adipogenesis of 3T3L1 cells through the S-sulfhydration of peroxisome proliferator-activated receptor 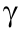 (PPAR
(PPAR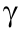 ). In their study, Verma et al. primarily use cultured preadipocytes from mice, which manifest characteristics similar to those of adipose tissues, thus providing a physiologically relevant model to study the complexity of adipose tissue. They demonstrate that H2S upregulates the insulin receptor β (Irβ)/mitogen-activated protein kinase (MAPK)/protein kinase B (Akt) pathway, leading to adipocyte differentiation, hypertrophy, and hyperplasia. The lack of endogenous H2S in CSE-KO mice suppresses adipogenesis and lipid accumulation in these animals. Under nutrient-overload and energy-imbalance conditions with high glucose and insulin over an extended period of time, H2S increases adipocyte hypertrophy, which is characterized by increased adipocyte diameter and lipid droplet size. The authors also observe that NaHS (an H2S salt) treatment increases glucose consumption in hypertrophic cells. These results demonstrate that H2S significantly increases adipogenesis and lipid accumulation and deteriorates obesity-related adipocyte hypertrophy under high-energy and nutrition-overload conditions. A better understanding of the role of H2S in obesity disorder will help in designing novel and mechanism-based safe therapeutic approaches to address obesity by selectively targeting the overproduced H2S in adipose tissues.
). In their study, Verma et al. primarily use cultured preadipocytes from mice, which manifest characteristics similar to those of adipose tissues, thus providing a physiologically relevant model to study the complexity of adipose tissue. They demonstrate that H2S upregulates the insulin receptor β (Irβ)/mitogen-activated protein kinase (MAPK)/protein kinase B (Akt) pathway, leading to adipocyte differentiation, hypertrophy, and hyperplasia. The lack of endogenous H2S in CSE-KO mice suppresses adipogenesis and lipid accumulation in these animals. Under nutrient-overload and energy-imbalance conditions with high glucose and insulin over an extended period of time, H2S increases adipocyte hypertrophy, which is characterized by increased adipocyte diameter and lipid droplet size. The authors also observe that NaHS (an H2S salt) treatment increases glucose consumption in hypertrophic cells. These results demonstrate that H2S significantly increases adipogenesis and lipid accumulation and deteriorates obesity-related adipocyte hypertrophy under high-energy and nutrition-overload conditions. A better understanding of the role of H2S in obesity disorder will help in designing novel and mechanism-based safe therapeutic approaches to address obesity by selectively targeting the overproduced H2S in adipose tissues.
Endoplasmic reticulum (ER) stress plays an important role in the development of metabolic syndrome due to its impact on apoptosis, autophagy, and cell proliferation. Yousof et al. have a special interest in the role of pleckstrin homology-like domain, family A, member 1 (PHLDA1) in ER stress-related metabolic diseases. In this issue, they review the current research status of the PHLDA1 gene and its location, function, and protein regulation, with a particular focus on the association of PHLDA1 with obesity, fatty liver diseases, diabetes, and atherosclerosis. ER stress upregulates the expression of PHLDA1 as a compensatory protection mechanism. Adipogenesis and white adipose tissue (WAT) expansion are inhibited by PHLDA1 through the inhibition of PPAR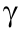 . A lack of PHLDA1 constitutes a causative factor in the development of insulin resistance and glucose intolerance in mice. The crucial regulatory role of PHLDA1 in various metabolic diseases is well-known. On the other hand, depending on cell type and disease state, PHLDA1 may alternate between pro- and antiapoptotic roles. As such, the therapeutic approach and targets based on PHLDA1 metabolism remain unsettled.
. A lack of PHLDA1 constitutes a causative factor in the development of insulin resistance and glucose intolerance in mice. The crucial regulatory role of PHLDA1 in various metabolic diseases is well-known. On the other hand, depending on cell type and disease state, PHLDA1 may alternate between pro- and antiapoptotic roles. As such, the therapeutic approach and targets based on PHLDA1 metabolism remain unsettled.
The metabolism of fatty acids is the primary source of the energy supply to the heart. However, fatty acids cannot be produced by the heart itself, and their availability relies on lipoprotein lipase (LPL), which breaks down circulating triglycerides into fatty acids. Following the onset of diabetes, LPL activity in the heart is enhanced to compensate for the reduction in glucose utilization for adenosine triphosphate (ATP) production. Unfortunately, this change also results in harmful lipid metabolite accumulation, fatty acid oxidation, and oxidative stress, leading to the development of diabetic cardiomyopathy (DCM). Lee et al. review the interconnections between LPL, endo-b-glucuronidase heparanase (Hpa), and vascular endothelial growth factor B (VEGFB), as well as their respective and related impacts on DCM development. The putative mechanisms underlying cardiac LPL changes are also discussed. Exploring the networks that connect vascular endothelial cell Hpa with cardiomyocyte LPL and VEGFB will provide clues to the homeostatic control of the energy balance between fattyacid- and glucose-based ATP productions in the heart, and will thereby advance the clinical treatment and management of DCM.
Wu et al. explore the relationship between exercise and cardiovascular disease (CVD) in patients with type 2 diabetes in China. They report that high levels of self-reported physical activity were associated with lower odds of CVD risk, and that reducing the sedentary time of older adults is critical to their physical health. The results of this study validate the health benefits of improving exercise regimens in patients with type 2 diabetes. Further studies are needed in order to compare the effects of different types of physical activity interventions in reducing CVD risk.
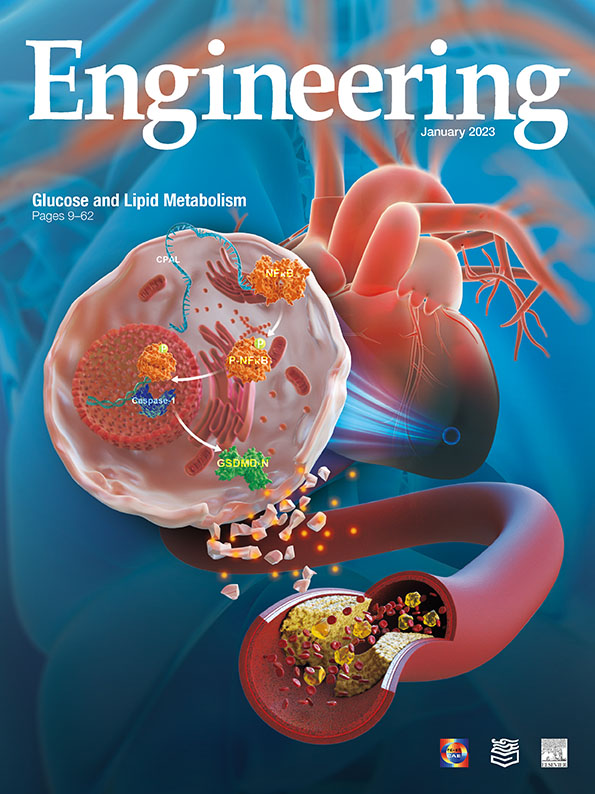
The role of a previously unknown conserved long noncoding RNA (lncRNA) AK009126 (CPAL) in myocardial infarction (MI) is unmasked by Li et al. in this issue. Multiple lines of evidence are provided to establish CPAL as a new regulator and biomarker for cardiac metabolic abnormalities and cardiomyocyte pyroptosis in MI. Thanks to this work, we now know that CPAL is a harmful lncRNA that impairs heart function. This study also leaves us with an intriguing question on the sequence of CPAL-mediated metabolic alterations and cardiomyocyte pyroptosis. CPAL may hold the key to improving cardiac dysfunction caused by ischemia/ inflammation. Moreover, this study provides a new direction for alleviating the complications of glucose and lipid metabolism. Reducing blood glucose and lipid levels can effectively improve the morbidity of CVD.
As the editors of this Special Issue, we are pleased to present the research discoveries reported in these five articles on the novel mechanisms and pathogenic targets of metabolic syndrome and its cardiovascular complications. The only way to win the battle against metabolic syndrome is to continuously and relentlessly explore, experiment, and extend our research efforts and scope. If this Special Issue helps in this collaborative and interactive approach and offers a platform for intellectual stimulation and debate, then its purpose is served; we hope that, by doing so, it will eventually play a role in benefiting people’s health and quality of life, as well as the welfare of our society.












 京公网安备 11010502051620号
京公网安备 11010502051620号




