《1. Introduction》
1. Introduction
The menstrual cycle is a series of natural processes that punctuate the life of women during their reproductive ages. It is conventionally divided into two cycling phases, the follicular and the luteal phases, which are defined by alternating estrogen (E) and progesterone (P) dominant periods intersected with short ovulatory and menstrual bleeding intervals [1]. The concentrations of sex hormones rise and fall during the menstrual cycle, causing the female body to exert different physiological and immune reactions that are highly dependent on the cycle phase [2].
During evolution, humans developed an innate and adaptive (humoral) immunity consisting of immune cells and molecules that stimulate immune reactions or provide various effector functions [3]. Among the immunoglobulins (IgG, IgM, IgA, IgD, and IgE), IgG is the most abundant human plasma glycoprotein and is an important effector molecule of humoral immunity. IgG is covalently modified with oligosaccharide chains called glycans through the complex co- and post-translational glycosylation process at Asn297, a conserved N-glycosylation site on each Fc portion of IgG heavy chains [4]. Glycans are essential structural and functional components, and are crucial regulators of IgG antibody’s effector functions [5,6]. Initiation of the immune response requires antigen recognition and IgG binding to various ligands, such as the Fc gamma receptors (Fc Rs) present on immune effector cells [7], complement factors [8], and lectins [9].
Rs) present on immune effector cells [7], complement factors [8], and lectins [9].
The IgG glycome composition is known to modulate the affinity of IgG for different ligands, thereby modulating its downstream effector functions. IgG glycans without a terminal galactose are associated with inflammatory and autoimmune disease pathology and act as inflammation initiators through the alternative complement pathway [10] or the lectin complement pathway [9]. On the other hand, galactosylated glycans are associated with decreased inflammatory antibody potential [11]. However, when galactosylated glycans lack a core fucose, they promote IgG binding to the C1q complement component and activate the inflammatory response, resulting in complement-dependent cytotoxicity (CDC) [12]. On average, over 90% of plasma IgG glycoforms have fucose on the glycan-core part, which acts as a ‘‘safety switch” on the inflammatory nature of IgG [13], as core-fucosylated glycans are 100-fold less efficient in activating antibody-dependent cellular cytotoxicity (ADCC) [14] than nonfucosylated glycoforms of the same IgG molecule [15]. Moreover, terminally sialylated glycans are unique in their ability to initiate anti-inflammatory effector functions by reducing IgG binding to activating Fc Rs [16] and promoting binding to type II (lectin) Fc
Rs [16] and promoting binding to type II (lectin) Fc Rs (e.g., DC-SIGN), while also stimulating the expression of inhibitory Fc
Rs (e.g., DC-SIGN), while also stimulating the expression of inhibitory Fc RIIB [17].
RIIB [17].
The composition of N-glycans on IgG changes with age, which was first noticed for galactosylated glycans [18]. Further studies have revealed gender differences in age-related changes in the galactosylation and sialylation of IgG, indicating that women have higher levels of digalactosylated and sialylated N-glycans than men, although such observations are reversed with menopause [19–21]. Moreover, a study involving thyroid cancer patients reported a sharp skew in IgG galactosylation in healthy female controls—particularly at puberty and menopause—and linked this observation with dramatic changes in the level of sex hormones [22].
An indication that sex hormones might be important modulators of pathological processes has been reported in regards to various autoimmune and inflammatory diseases in women [23]. Many studies on disease mechanisms attribute a significant role to altered IgG N-glycosylation [24]. Evidence of the relationship between sex hormones and IgG N-glycosylation was provided by studies comparing disease activity in women before, during, and after pregnancy [25–27], examining the increased disease risk associated with endogenous postmenopausal sex hormone levels [20], or comparing the disease incidence in men and women of the same age [28–31]. Very low plasma estrogen levels are associated with an increased risk of rheumatoid arthritis development in menopause [32,33]. Moreover, a direct causal relationship between sex hormones and altered IgG glycosylation has been shown in endocrine intervention studies [32,34]. Our group recently discovered that the chronic suppression of gonadal hormones in healthy menstruating women increased the glycan age index [20], a newly developed glycan biomarker of biological age, which aligns well with lifestyle and disease-risk biomarkers and increases with the increasing abundance of IgG glycans without galactose and sialic acid [35]. Inversely, high estrogen was shown to promote IgG galactosylation and sialylation, causing disease remission in pregnant rheumatoid women [27]. As for the menstrual cycle, premenopausal women generally experience worsening chronic disease symptoms in the premenstrual period or during menses when sex hormones are low [2].
The abovementioned research indicates a possibility that the fluctuation of sex hormones during the menstrual cycle might affect IgG N-glycosylation and consequently affect a woman’s immunity. Since changes in IgG glycosylation are concomitant factors of ageing, health, and sex hormone status, glycans have become inevitable subjects in an increasing number of epidemiological studies [24]. Glycans have been demonstrated to be contributors to and predictors of disease risk and development [36] and have the potential to become widely used diagnostic biomarkers [37,38]. Thus, it is imperative to thoroughly investigate all factors that might affect the N-glycosylation of IgG.
There is as yet no data on possible IgG N-glycosylation changes during the menstrual cycle, nor is it known whether the menstrual cycle phase should be considered a confounder in epidemiological studies. Therefore, this study examined longitudinal changes in IgG glycome composition during the menstrual cycle in a cohort of healthy premenopausal women. We also checked whether an abundance of IgG glycan traits was associated with menstrual cycle phases and with the concentration of sex hormones in plasma.
《2. Materials and methods》
2. Materials and methods
《2.1. Participants》
2.1. Participants
This study recruited female volunteers through a project titled ‘‘The association between the glycosylation of IgG and the female menstrual cycle,” financed by Capital Medical University in Beijing, China. The exclusion criteria included: confirmed menopause state; disease or sickness preceding the study; usage of oral contraceptives, other hormonal substances, or medications; cigarette smoking; alcohol consumption; and pregnancy. All these factors are known to affect the N-glycosylation of IgG [39]. A total of 70 premenopausal women aged 19–48 years with regular menstrual cycles met the criteria for the survey, questionnaire 1 (Appendix A), and were eligible to participate in the study (Table S1 and Fig. S1 in Appendix A).
《2.2. Study design》
2.2. Study design
This was an observational study in which the participants were purposely sampled over 12 consecutive weeks from September to November 2016. Blood samples were collected once a week (every 7 days) for 3 months, regardless of the menstrual cycle phase (12 samples per person). A study design chart with an overview of the sampling protocol and subsequent experimental procedures is depicted in Fig. S2 in Appendix A. In short, plasma was separated from each blood sample and stored at –80 °C until sex hormone and IgG N-glycan analysis (details are provided in Appendix A). During the weekly visits, the participants underwent phenotypic measures and a follow-up survey, questionnaire 2 (Appendix A), in which they were asked to report their menstrual cycle details since the previous blood withdrawal. This information was used to determine the menstrual cycle length for each participant during the 12-week study period (Table S2 in Appendix A).
《2.3. Sex hormones》
2.3. Sex hormones
Plasma samples were analyzed to determine the concentrations of three sex hormones—17β-estradiol (E2), progesterone (P), and total testosterone (T)—using commercially available immunoassay kits (ARC Estradiol RGT, ARC Progesterone RGT, and ARC 2nd Gen Testo RGT, Abbott, Abbott Laboratories, USA). Sex hormone plasma concentrations were measured in a single reading using an Architect i1000SR chemiluminescence immunoassay analyzer (Abbott, Abbott Laboratories, USA), and Architect System software was used for automated data analysis.
《2.4. IgG isolation, N-glycan release, and labeling》
2.4. IgG isolation, N-glycan release, and labeling
The whole procedure was performed according to the protocol established by Pucic´ et al. [40]. In short, IgG was isolated from the plasma samples by means of affinity chromatography on a 96-well Protein G plate (BIA Separations, Slovenia). The amount of IgG in each sample was measured on a NanoDrop 8000 Spectrophotometer (Thermo Fisher) after every IgG isolation procedure (aver. IgG conc. = 0.7 mg∙mL–1 ; range: min. IgG conc. = 0.36 mg∙mL–1 , max. IgG conc. = 1.1 mg∙mL–1 ). The isolated IgG was denatured by adding sodium dodecyl sulfate (SDS; Invitrogen, USA) and incubating at 65 °C. Igepal-CA630 (Sigma-Aldrich, USA) was used to neutralize the excess SDS. IgG N-glycans were released by adding PNGase F (Promega, USA) in phosphate-buffered saline (PBS), followed by overnight incubation at 37 °C. The released N-glycans were fluorescently labeled with a 2-aminobenzamide (2- AB) dye (Merck, Germany). The free label and reducing agent were removed from the samples using hydrophilic interaction liquid chromatography solid-phase extraction (HILIC-SPE). The IgG N-glycans were eluted with ultrapure water and stored at –20 °C until use.
《2.5. IgG N-glycan analysis》
2.5. IgG N-glycan analysis
Labeled N-glycans were separated by means of hydrophilic interaction liquid chromatography (HILIC) on a Waters Acquity ultra-performance liquid chromatography (UPLC) system (Waters, USA) composed of a sample manager, quaternary solvent manager, and fluorescence detection (FLR). An amide Acquity UPLC Glycan BEH chromatography column (Waters, USA), with 100 mm 2.1 mm (i.d.) and 1.7 μm ethylene-bridged hybrid particles, was used to separate the N-glycans, with 100 mmol∙L–1 ammonium formate at pH 4.4 as solvent A and 100% acetonitrile (ACN) as solvent B. Samples were kept at 10 °C before injection into the column, and the separation itself was performed at 60 °C. The separation method used a linear gradient of 75%–62% ACN at a 0.40 mL∙min–1 flow rate in a 27 min analytical run. N-glycans were detected by an FLR detector with excitation and emission wavelengths set at 250 and 428 nm, respectively. The system was controlled by Empower 3 software, build 3471 (Waters, USA).
Data processing included a build-in integration of chromatograms, after which the chromatograms were manually corrected to maintain the same intervals of integration for all the samples. Selected chromatograms were used to template the automated integration of the chromatographic glycan data of all samples [41]. The chromatograms were equally separated into 24 glycan peaks (GP1–GP24) that contained different glycan structures (Fig. 1) [19,42]. The N-glycan composition in chromatographic peaks was assigned previously by Pucic´ et al. [40]. The abundance of glycans in each peak was expressed as a percentage of the total integrated area (% area).
《Fig. 1》
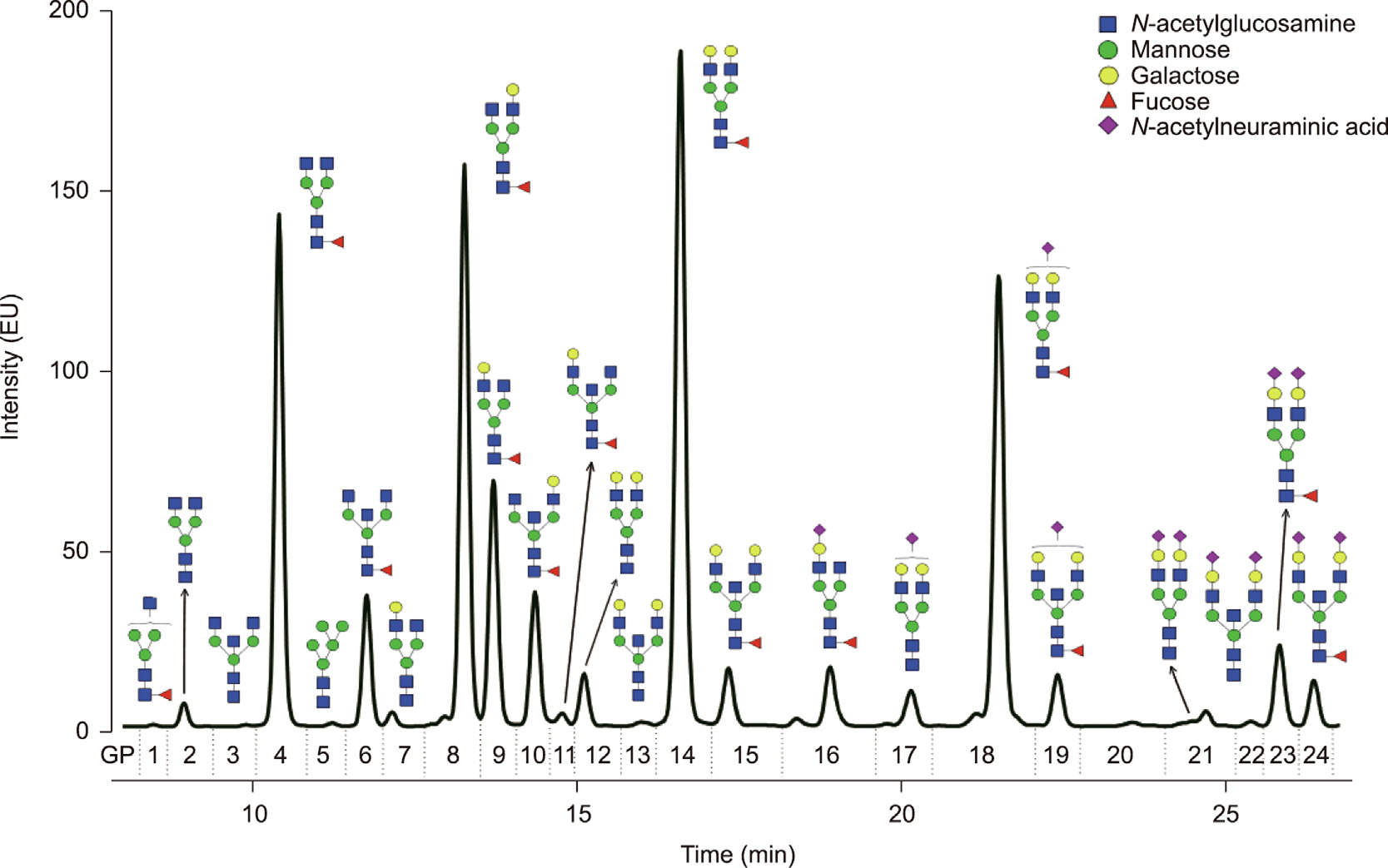
Fig. 1. Human IgG N-glycome. Representative chromatogram of HILIC-UPLC-FLR-analyzed 2-AB labeled N-linked glycans released from plasma-derived IgG separated into 24 chromatographic peaks (GP1–GP24). Only the most abundant glycoform is presented for each glycan peak to simplify the chromatographic data presentation. A complete list of glycan structures in human IgG N-glycome is provided in Ref. [19]. Monosaccharide residues are graphically presented by geometrical symbols and color-coded according to the Consortium for Functional Glycomics recommendation. Glycan structures were depicted using GlycoWorkbench software [42]. EU: emission units; GP: glycan peak.
《2.6. Statistical analysis》
2.6. Statistical analysis
To avoid the batch effect that arises when analyzing multiple samples at different times and on different plates, all samples collected from one woman were randomly distributed over a single 96-well plate and analyzed in the same run. Following this logic, we distributed plasma samples from three to five age-matched individuals and an in-house plasma sample (standard) per plate. As a standard, we used plasma from a healthy anonymous female donor (32 years of age) collected at a single timepoint by the Department of Transfusiology, Zagreb, Croatia. The standard was distributed in a pentaplicate over all plates to control for nonbiological variation—that is, the technical variation of the method. UPLC glycan data were normalized by the total chromatographic area to make measurements across samples comparable. Each glycan peak was quantified as a percentage of the total IgG N-glycome.
The biological variation of each glycan peak was calculated as a ratio of the median variation of the standard (technical variation) and median of the variations in all samples for every woman in the cohort for each of the 24 glycan peaks (variable) multiplied by 100% (Table S3 in Appendix A). Aside from 24 directly measured glycan peaks, for IgG glycans, six additional derived traits were calculated as a sum of selected glycan peaks with similar structural features (Table 1). The derived traits represent the relative abundance of agalactosylated glycans (G0), monogalactosylated glycans (G1), digalactosylated glycans (G2), sialylated glycans (S), corefucosylated glycans (F), and N-glycans with bisecting GlcNAc (B).
《Table 1》
Table 1 Derived IgG glycan traits defined from 24 directly measured glycan peaks (Fig. 1).
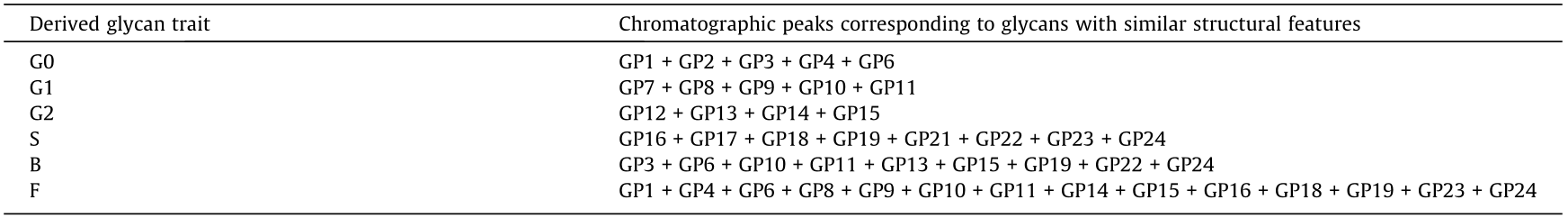
G0: glycans with no galactose (agalactosylated); G1: glycans with one galactose (monogalactosylated); G2: glycans with two galactoses (digalactosylated); S: glycans with sialic acid (sialylated); B: glycans with bisecting N-acetylglucosamine (GlcNAc) (bisected); F: glycans with fucose on the most inner GlcNAc (core-fucosylated).
To assess the dynamics of glycans and sex hormones in the menstrual cycle, we built a menstrual cycle model by normalizing the length of each menstrual cycle in the study to 100%. The model was built from the glycan and sex hormone data of 500 analyzed plasma samples in 140 menstrual cycles from 60 women (for details, see Section 3.2). Association analyses between the menstrual cycle and IgG glycan traits were performed using a linear mixed model with age (fixed effect) and subject (random effect) included as additional covariates. The assumed periodic pattern of longitudinal glycan measurements was modeled as a linear combination of sine and cosine functions of the menstrual cycle phases. Association analyses between the concentration of each sex hormone and IgG glycan traits were performed using a linear mixed model with age (fixed effect) and subject (random effect) included as additional covariates.
All glycan measurements were standardized by dividing each glycan trait by its average within an individual during the whole study duration, making estimated effects across the menstrual cycle comparable between different glycan traits and between individuals. The Benjamini–Hochberg method was used to adjust the p values for the number of hypothesis tests performed. A p value < 0.05 was considered to be statistically significant. Data were analyzed and visualized using the R programming software (version 3.0.1).
《2.7. Institutional review board statement》
2.7. Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Capital Medical University (CMU), Beijing, China (protocol code 2016SY106; approved on 2017 Jul 11) and by the Ethics Committee of the School of Medicine at University of Zagreb, Croatia (protocol code 380-59-10106-18-111/151; approved on 2018 Jul 12). The participants provided written informed consent according to the Declaration of Helsinki.
《2.8. Informed consent statement》
2.8. Informed consent statement
Informed consent was obtained from all subjects involved in the study.
《3. Results》
3. Results
《3.1. Study overview》
3.1. Study overview
We performed longitudinal N-glycoprofiling of plasma-derived IgG in 70 healthy premenopausal women with regular, natural menstrual cycles, with the aim of investigating possible associations between menstrual cycle phases and IgG N-glycan composition. We followed changes in IgG glycome composition over 12 consecutive weeks, with a once-a-week sample collection rate, regardless of the menstrual cycle phase. Seven participants did not complete the full sampling study protocol, and one participant entered the sampling protocol from the third week; thus, fewer than the initially planned 12 plasma samples per person were obtained for these individuals. The sampling protocol resulted in 806 plasma samples available for experimental procedures (Fig. S2).
《3.2. Menstrual cycle selection criteria》
3.2. Menstrual cycle selection criteria
To define the length of the menstrual cycles in our study, selfreported information on menstrual cycles was used, obtained through survey questionnaires completed by each participant before entering the study and during the study at each subsequent blood withdrawal. We noticed that some menstrual cycles went out of the normal cycle ranges [43], which was considered to be an exclusion criterion. Finally, the study-specific acceptable average menstrual cycle length of (30 ± 4) days was established, resulting in 500 UPLC IgG glycan datasets in 140 menstrual cycles from 60 women for further statistical analysis (Table S2).
《3.3. Assessment of IgG N-glycosylation variability》
3.3. Assessment of IgG N-glycosylation variability
Before assessing the extent of the IgG N-glycosylation changes in the menstrual cycle, we first had to determine the biological variability of individual IgG glycans. We compared the variation of each glycan peak in all analyzed samples in the cohort with the analytical variation of the standard sample for the entire study period. We found that the biological variation exceeded the analytical variation in 14 out of 24 glycan peaks (Table S3), which are the major peaks comprising roughly 85% of the total chromatographic area, as determined by Bakovic´ et al. [19].
We determined the interpersonal variability of the IgG N-glycosylation in the cohort represented by an interquartile range (25th–75th percentiles) of derived glycan traits in all analyzed samples, as described in Table S4 in Appendix A. We found the most prominent variation in the level of galactosylation-related traits, especially agalactosylation (G0) (s = 4.3, where s stands for standard deviation), while fucosylation (F) had the lowest variability range in all samples in the cohort (s = 0.9) (Fig. 2(a)).
《Fig. 2》

Fig. 2. Variability in the N-glycosylation of IgG. (a) Interpersonal variability of N-glycosylation in the cohort; a box plot for the relative abundance of six derived IgG glycan traits in all samples analyzed in the cohort. Each box represents the interquartile range (25th–75th percentile). Lines inside the boxes represent the median (x~), while whiskers represent the 5th and 95th percentiles. Dots indicate outliers. For each derived trait, the mean (x) and standard deviation (s) were also calculated (Table S4). (b) Intrapersonal variability of N-glycosylation for a single person. Each blue vertical line represents the variation range defined by the min and max values of six derived IgG glycan traits for each female (n = 60) over a 12-week study period. Black vertical lines represent the variation range of glycan traits for the standard, which is an in-house sample with a known N-glycosylation profile used to follow technical variation. Glycan measurements were standardized by their average within an individual to enable comparison between different glycan traits.
Next, we assessed the variation of derived IgG glycan traits for each person. Fig. 2(b) shows that the extent of intrapersonal variation was the most pronounced for sialylated (S) (with the largest difference between min and max values being up to 21%) and agalactosylated (G0) (with the largest difference between min and max values being up to 16%) IgG N-glycans. In comparison, core-fucosylated (F) IgG N-glycans showed the smallest variation within a single female (with the largest difference between min and max values being less than 3%).
《3.4. IgG N-glycosylation shows a cyclic periodic pattern in the menstrual cycle》
3.4. IgG N-glycosylation shows a cyclic periodic pattern in the menstrual cycle
We tested for associations between menstrual cycle phases and derived glycan traits to characterize the detected IgG Nglycosylation changes in the menstrual cycle. Our results revealed a cyclic periodic nature in the abundance dynamics for five out of six IgG N-glycosylation traits: agalactosylation (G0), monogalactosylation (G1), digalactosylation (G2), sialylation (S), and bisecting GlcNAc (B) (Fig. 3, Table 2). The average variation in the abundance due to the cycle phase was relatively small—only up to 1.1%. Table 2 shows that the derived glycan traits related to the galactosylation and sialylation of IgG had the highest variation: G0 was 1.1%, and G2 and S were 1.0%. N-glycans with one galactose were moderately variable—G1 was 0.8%, as well as N-glycans with bisecting GlcNAc B was 0.5%. The fucosylation (F) level remained stable and did not show statistically significant cyclic periodicity during the menstrual cycle (Table 2).
《Table 2》
Table 2 Associations of menstrual cycle phases with derived IgG glycan traits and analysis of derived glycan traits variation resulting from the menstrual cycle and interpersonal differences in IgG N-glycosylation.
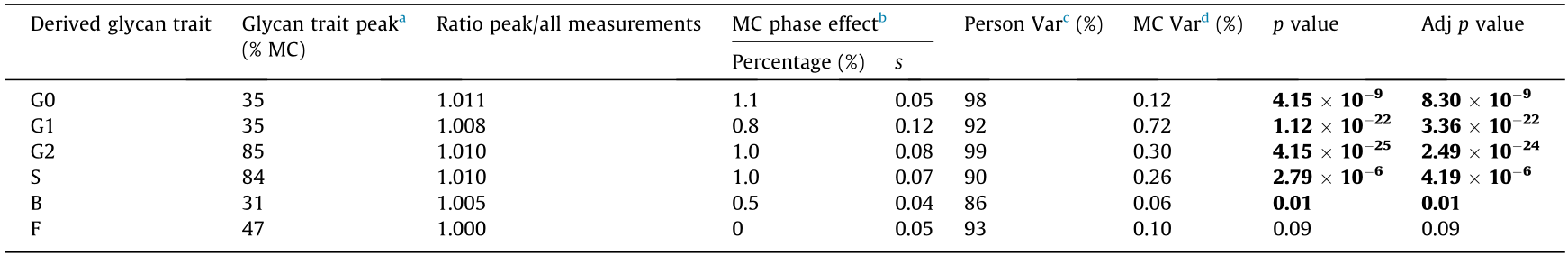
p values describe the statistical significance of derived glycan trait abundance variability introduced by the menstrual cycle. False discovery rate was controlled using the Benjamini–Hochberg procedure (p-value adjusted). Statistically significant adjusted p (Adj p) values are in bold (p < 0.05). The duration of one MC corresponds to 100%. Follicular phase range: 0%–50% MC; luteal phase range: 50%–100% MC. MC: menstrual cycle.
a The point of the menstrual cycle phase at which the highest abundance of glycans with a similar structural feature, i.e., derived glycan trait, was observed.
b Ratios of an average peak trait measurement and an average of all trait measurements for each derived glycan trait are shown as effects of the menstrual cycle phase on the variability of derived glycan trait abundance, expressed in percentages (%) and standard deviations (s).
c Percentage of derived glycan trait variability explained by interpersonal differences (Person Var) in IgG N-glycosylation (%).
d Percentage of derived glycan trait variability explained by the menstrual cycle (MC Var; %).
We were also able to distinguish two different patterns of N-glycosylation changes across the menstrual cycle. We noticed the highest abundance of G0, G1, and B in the follicular phase of the menstrual cycle, while G2 and S reached the highest abundance in the luteal phase of the menstrual cycle, as shown in Fig. 3.
《Fig. 3》
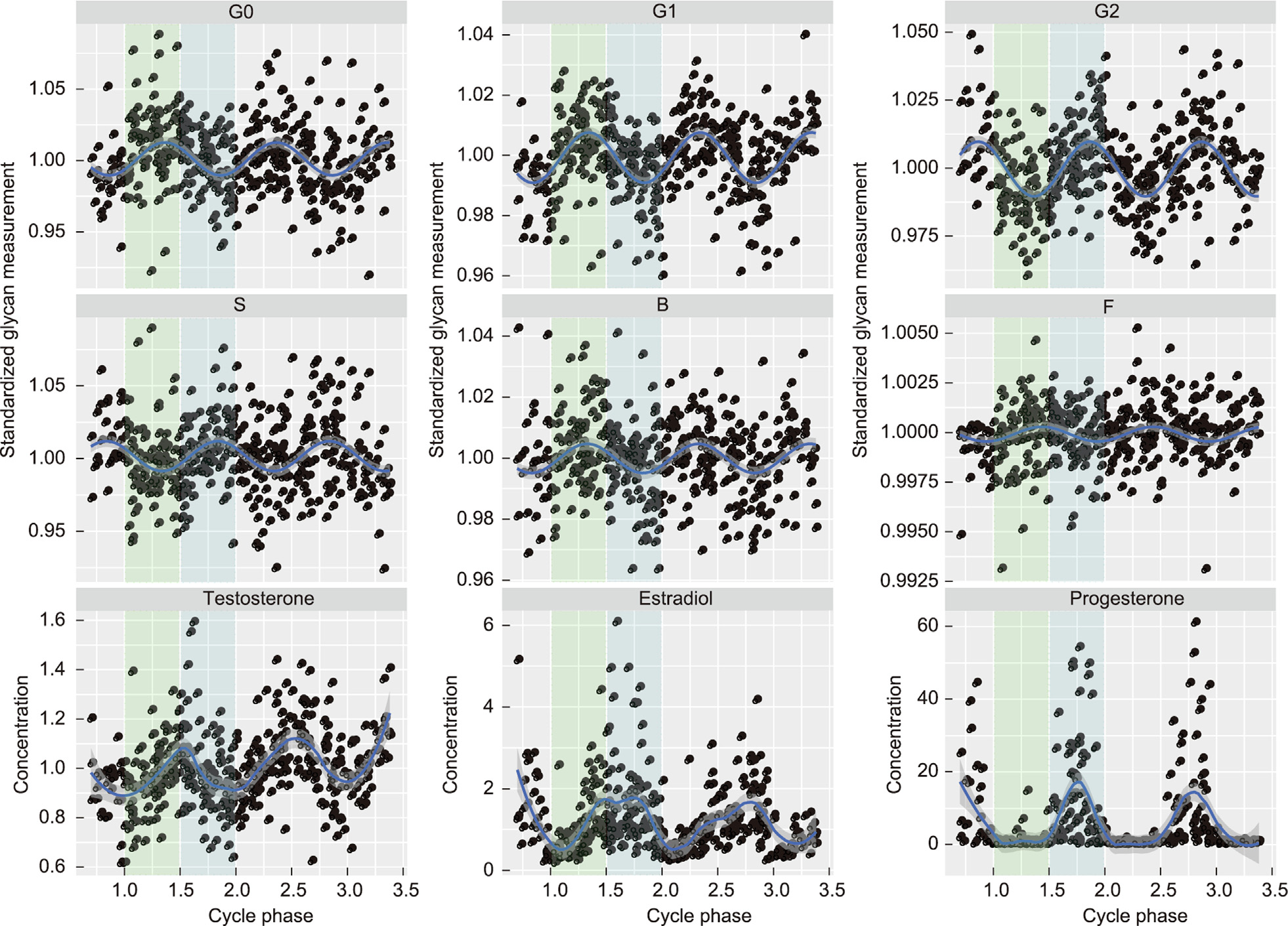
Fig. 3. Dynamics of IgG N-glycosylation and sex hormones during several consecutive menstrual cycles. The blue curve represents the modeled function, and the dark grey area represents the 95% confidence interval of variations in N-glycans with no galactoses (G0), one galactose (G1), two galactoses (G2), sialic acid (S), bisecting GlcNAc (B), and core fucose (F), and the plasma concentrations of three sex hormones—testosterone (nmol∙L–1 ), estradiol (pmol∙L–1 ), and progesterone (nmol∙L–1 )—through the follicular and luteal phases of several menstrual cycles (as shown in Table 3). The integers on the x-axis represent the beginning of each subsequent menstrual cycle. Glycan measurements were standardized by their average within an individual (see Section 2.6) to enable comparison between different glycan traits. Green rectangle: follicular phase (shown for the first menstrual cycle observed only); blue rectangle: luteal phase (shown for the first menstrual cycle observed only); each dot represents one sample; n(samples) = 500.
Next, we examined the extent to which the menstrual cycle contributes to the overall variation of IgG N-glycosylation. Linear mixed model analysis with age as a fixed variable and the subject as a random variable showed that the menstrual cycle could explain as little as 0.06% of the variability in the abundance of N-glycans with bisecting GlcNAc (B) (adjusted (Adj) p = 0.01) to at most 0.72% of the variability in the abundance of monogalactosylated (G1) (Adj p = 3.36 × 10–22) N-glycans. Therefore, the results show that changes in IgG N-glycosylation resulting from the menstrual cycle alone account for less than 0.8% of the abundance variability in any glycan trait for all samples in the cohort. By comparison, over 86% of the variability in the abundance of bisected N-glycans (B) detected on IgG could be attributed to differences in N-glycosylation between individuals. Moreover, the abundance of digalactosylated N-glycans between two people may differ by as much as 99% for the same reason (Table 2).
《3.5. Peak alterations in IgG N-glycosylation and the highest concentration of sex hormones are time-shifted across the menstrual cycle》
3.5. Peak alterations in IgG N-glycosylation and the highest concentration of sex hormones are time-shifted across the menstrual cycle
To explore the known links of sex hormones—notably estrogens—with changes in IgG N-glycosylation [32,34], we first wanted to check whether we could match the peak plasma concentrations of herein-studied sex hormones with the peak abundances of derived glycan traits. The relationship between the highest concentration of sex hormones in plasma and the highest level of derived IgG glycan traits across the menstrual cycle is given in Table S5 in Appendix A.
We observed that the literature-based highest estradiol concentration in the follicular phase [44] does not overlap with the highest abundance of G2 or S glycans, although it coincides with the estradiol and progesterone peak in the luteal phase of the menstrual cycle, as shown in Fig. 3. Our study demonstrates that the G2 and S peaks occur at approximately the 25th day (82% MC, where MC stands for menstrual cycle) in the luteal phase, 12 days after the literature-based highest estradiol concentration at approximately the 13th day (45% MC) of the follicular phase in the menstrual cycle. Moreover, we observed IgG digalactosylation and sialylation peaks 9 days after the highest testosterone concentration at approximately the 16th day (55% MC) of the menstrual cycle and concurrent with the highest progesterone and estradiol concentration in the luteal phase of the menstrual cycle. On the other hand, the highest abundance of agalactosylated N-glycans, monogalactosylated N-glycans, and N-glycans with bisecting GlcNAc showed no overlap with any of the points in the menstrual cycle at which the highest concentrations of sex hormones were observed. Instead, we saw that the bisection, agalactosylation, and monogalactosylation of IgG reached a peak on the 9th (31% MC) and 10th (35% MC) days of the menstrual cycle during the early follicular phase, which is characterized by low (progesterone) to moderate (estradiol and testosterone) levels (Fig. 3).
《3.6. Plasma concentrations of sex hormones are associated with IgG N-glycosylation pattern》
3.6. Plasma concentrations of sex hormones are associated with IgG N-glycosylation pattern
After discovering a mismatch between the IgG N-glycosylation pattern and the fluctuation of sex hormones during the menstrual cycle, we checked for possible associations between the sex hormones and the derived IgG glycan traits. The associations of the peak concentrations of all three herein-studied sex hormones with the peak abundances of different IgG glycan traits are shown in Table 3.
《Table 3》
Table 3 Associations of sex hormones with derived IgG glycan traits during the menstrual cycle.
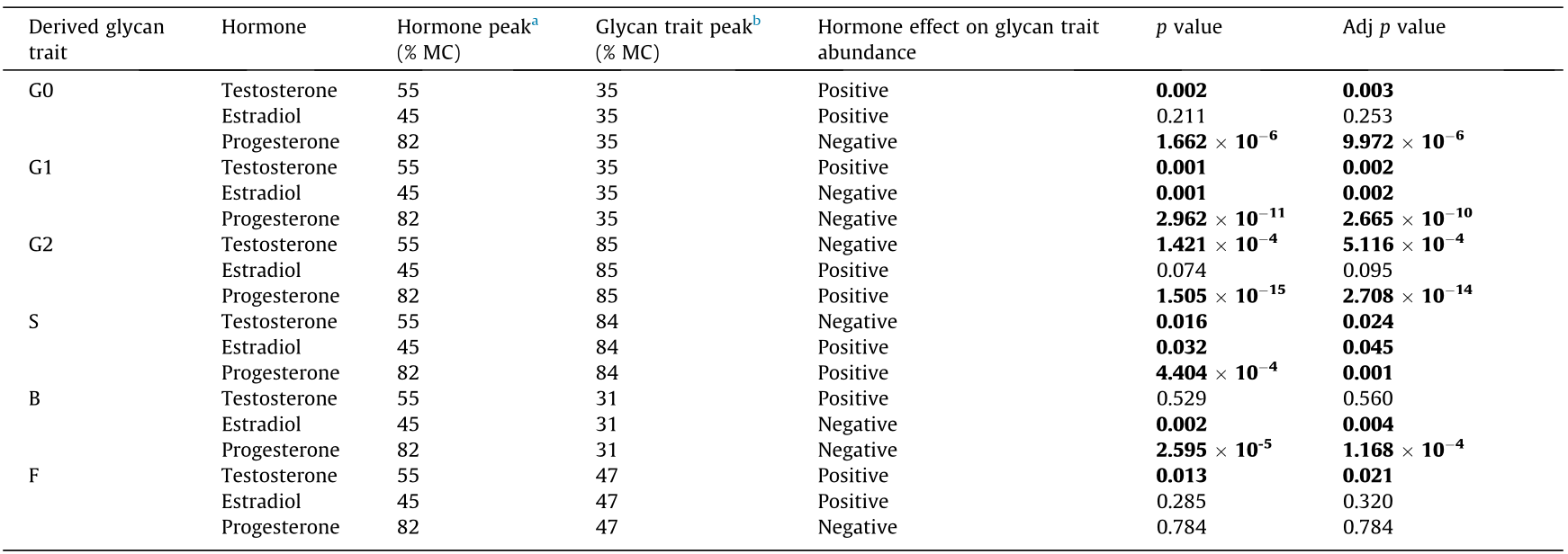
p values describe the statistical significance of associations between peak concentrations of sex hormones in plasma and peak abundances of derived glycan traits. Associations are shown as the functional effect of sex hormones on derived glycan trait abundance. The false discovery rate was controlled using the Benjamini–Hochberg procedure (p-value adjusted). Statistically significant Adj p values are in bold (p < 0.05). The duration of one MC corresponds to 100%. Follicular phase range: 0%–50% MC; luteal phase range: 50%–100% MC.
a The point of the menstrual cycle phase at which the highest serum concentration of analyzed sex hormone was observed.
b The point of the menstrual cycle phase at which the highest abundance of glycans with a similar structural feature, i.e., derived glycan trait, was observed.
We saw that progesterone and estradiol manifest similar associations with IgG N-glycosylation during the menstrual cycle. Estradiol is associated with increased sialylation, while progesterone—in addition to sialylation—is associated with increased digalactosylation of IgG. Moreover, both estradiol and progesterone are inversely associated with IgG glycans with bisected GlcNAc or one galactose unit, while agalactosylated IgG glycans are inversely associated solely with progesterone concentrations in plasma. Testosterone has been found to have quite the opposite association with the N-glycosylation of IgG compared with progesterone and estradiol: Testosterone is associated with increased agalactosylated, monogalactosylated, and core-fucosylated IgG glycans during the menstrual cycle.
《4. Discussion》
4. Discussion
We analyzed IgG glycosylation patterns in the menstrual cycles of 70 healthy eumenorrheic women by collecting blood weekly for 12 weeks, spanning roughly three menstrual cycles. Our study demonstrated that the composition of IgG glycome went through modest periodic changes during menstrual cycles, which were associated with female sex hormones and menstrual cycle phases. However, peak alterations of individual IgG glycan traits did not overlap with the highest concentrations of sex hormones in plasma but appeared as menstrual cycle phase-specific events. Furthermore, we saw that sialylation and agalactosylation were the most variable IgG glycan traits within a single female and on the cohort level. Given that the N-glycosylation of IgG can impact antibody–ligand interactions and, hence, immune responses, studies aiming to understand the hormonal regulation of IgG glycosylation are essential to our understanding of women’s health.
Previously published data on the effects of sex hormones on IgG N-glycosylation in women were deduced from epidemiological studies dealing with pathological mechanisms, either in various diseases [23] or during physiological states such as pregnancy [25] and menopause [32], which alter with the concentration of sex hormones. Our present data support the hypothesis that sex hormones are relevant to IgG N-glycosylation in healthy premenopausal menstruating women. During the menstrual cycle, there is an alternation between reproductively unfavorable periods of intense immune activity and reproductively favorable periods with reduced immune reactions, regulated by estrogen and progesterone [45]. Estrogen is known to have both inflammatory and anti-inflammatory properties, which depend on its concentration (where a high concentration has anti-inflammatory activity) and on the ratio between estrogen and progesterone concentrations in the body [46], while progesterone dominantly exerts antiinflammatory actions [47]. These findings suggest that sex hormone fluctuations during the menstrual cycle might affect IgG Nglycosylation and, consequently, cellular [2] and humoral immunity in women [48].
Moreover, we specified the dynamics of IgG N-glycosylation changes as cyclic periodic variations of relative abundances in most glycan traits. We showed that the first part of the menstrual cycle—the follicular phase—is characterized by the highest abundance of agalactosylated glycans, monogalactosylated glycans, and glycans with bisecting GlcNAc, which are known to be associated with inflammatory IgG activity [49], while, after the ovulation, there is an increasing abundance of anti-inflammatory digalactosylated and sialylated N-glycans [11,16]. Similar results were found in studies reporting the coexistence of high estrogen and progesterone levels with anti-inflammatory-oriented antibodies produced by the pregnancy-modulated female immune system [50,51]. Previous studies revealed that interpersonal differences in the human IgG N-glycome on the population level show significant variability in glycan abundances [40,52], with galactosylation as the most variable of traits [53,54], which is confirmed by our study. On the other hand, the relative abundance of fucosylated N-glycans did not cyclically change with the menstrual cycle. This is not surprising, because IgG fucosylation remains stable through adulthood [19], and significant changes occur only with autoimmune diseases and cancer [55,56]. Our cohort was comprised of healthy women who were not challenged with pathogens or had any recorded changes in their health status during the study sampling period. In addition, thus far, no association between changes in total IgG fucosylation and sex hormones has been reported in the literature. Thus, the observation of minimal variations in fucosylation level during the menstrual cycle aligns with our expectations.
Based on the above-described findings, we assumed that the cyclic nature of the IgG N-glycosylation pattern might be an effect associated with a fluctuation of sex hormones repeated through monthly menstrual cycles. In this context, the day of the menstrual cycle may act as a confounder for assessing the variation in the Nglycosylation of IgG [57]. Although the peak alterations of glycan trait abundances were associated with the menstrual cycle phases, they resulted in the minimal average variation of any glycan trait across the menstrual cycle in the cohort, as shown by our model. Moreover, the variations in IgG N-glycosylation related to the menstrual cycle seemed very small compared with changes introduced by disease or age, which were previously examined by our group [58]. The reason for the small effect of hormones on the Nglycosylation of IgG during the menstrual cycle of healthy young females might come from the fact that IgG has a long half-life of 3 weeks [59]. This means that the dynamic of sex hormone fluctuation during the menstrual cycle is faster than the production and glycosylation of new IgGs. Therefore, the glycosylation of IgG most probably reflects the average sex hormone levels in a time period that exceeds the length of one menstrual cycle. To conclude, the observed trend suggests that the N-glycosylation of IgG in menstruating women depends on the menstrual cycle day, although this effect is minimal in the context of studies involving a large number of participants.
For the first time, our study showed a shift between the peak alterations of glycan trait abundances and the peak concentration of individual sex hormones during the menstrual cycle. A manifestation of the sex hormonal effect on IgG N-glycosylation shifted because IgG has a long half-life (21 days) compared with the duration of the menstrual cycle. This postulates that IgG production and N-glycosylation might be initiated by earlier stimulations of the immune system [60], perhaps through fluctuating sex hormone concentrations preceding the ongoing menstrual cycle.
Hormone intervention studies on the effect of estrogen on IgG glycosylation are consistent [32,34]. Our study implicates the relevance of anti-inflammatory estradiol properties by revealing a positive association of estradiol concentration with the abundance of sialylated N-glycans, which might result from the upregulation of β-galactoside α-2,6-sialyltransferase 1 (St6Gal1)—the key sialyltransferase for adding sialic acids to IgG in B-lineage cells[32]. Recently, Jones et al. [61] reported the existence of a quick alternative B-cell-independent sialylation of IgG in the bloodstream, which cannot be solely attributed to estradiol, since our study revealed estradiol and sialylation peaks appearing in different phases of the menstrual cycle. It is known that galactosylated glycans are the substrates for subsequent terminal IgG sialylation [62]. In line with this, we noticed the same direction of change in the sialylation and digalactosylation patterns of IgG throughout the menstrual cycle. This might imply the existence of a mutual regulatory mechanism of IgG galactosylation and sialylation in the cell secretory pathway [63], possibly through heterodimer complex formation between β-1,4-galactosyltransferase (B4GalT1), which adds galactoses, and St6Gal1, which adds sialic acids [64]. The enzymatic activity of these two glycosyltransferases could be modified by estrogen receptors that, upon E2 binding, act as nuclear transcription factors initiating the expression of specific genes as part of the immune system activity [65].
Even though estrogen has been reported to affect IgG galactosylation [34], there is still no clear answer on the specific mechanism. A genome-wide association study (GWAS) from 2020 [66] identified the genes coding for the transcription factors RUNX1, RUNX3, SPINK4, and ELL2 as potential participants in downstream signaling mechanisms linking E2 with IgG galactosylation, as later shown by in vitro experiments conducted by Mijakovac et al. [67]. Our study did not show significant associations between estradiol and digalactosylated glycans directly, indicating that estradiol might be generally relevant but presumably not a single critical factor in regulating IgG galactosylation level during the menstrual cycle.
Studies evaluating the effect of other hormones on protein glycosylation are scarce [68]. Intervention studies on progesterone [69] and testosterone [70] are rare but suggest an association with IgG N-glycosylation levels; therefore, the effect of these hormones on IgG N-glycosylation has yet to be investigated. In vivo modulation of IgG galactosylation by testosterone has already been found to reflect the action of testosterone-derived estradiol rather than testosterone itself [34]. We observed associations between testosterone and almost all IgG glycan traits. This is not surprising, since testosterone plays a critical role in the development of mature B and T lymphocytes through androgen receptors that function as ligand-activated transcription factors, altering general gene expression [71] and presumably also affecting the expression of genes that code for enzymes involved in protein N-glycosylation processing within B cells. Previous research on prostate cancer by Munkley et al. [70] revealed a possible mechanism by which testosterone expresses anti-inflammatory N-glycosylation immune properties by down-regulating sialyltransferase activity, thus lowering cancer cell viability. Even though studies on men point out the anti-inflammatory [72] and autoimmune protective properties of testosterone through the restriction of B cell number [73], excess testosterone in women is associated with chronic lowgrade inflammation, which is an underlying pathological mechanism in the sex hormone disorder of polycystic ovary syndrome (PCOS) [74]. According to these studies, our present data highlight the relevance of testosterone in the overall inflammatory IgG phenotype in women through its positive association with inflammatory glycoforms and inverse association with anti-inflammatory glycoforms. Still, in healthy menstruating women, ovarianproduced testosterone provides an abundant reservoir from which the most potent estrogen—estradiol—is subsequently synthesized by the action of the aromatase enzyme [75,76], making testosterone irreplaceable in the normal functioning of the female reproductive system.
Progesterone also shows solid immunoregulatory properties [77], especially regarding immunological tolerance—the most critical feature of successful pregnancies [78]. Our study implicates the relevance of progesterone to anti-inflammatory IgG activity during the luteal phase of the menstrual cycle, presumably arising as a physiological effect on the immune system extending into pregnancy [78]. Progesterone actions on immunity probably employ different mechanisms that involve the modulation of immune cells [79] and antibody functionality [69,80], as well as hindering pro-inflammatory cytokine production [81], resulting in the domination of less inflammatory antibodies. Our research shows a statistically significant progesterone association that perhaps outweighs the relevance of estradiol and testosterone to IgG N-glycosylation.
We presume that the mechanistic aspect of the hormonal regulation of IgG glycosylation in the menstrual cycle might be explained by linking sex hormones with glycogenes’ activity. Glycogenes are genes encoding the enzymes directly involved in IgG N-glycosylation (e.g., glycosyltransferases, glycosidases, and sugar nucleotide transporters); there are estimated to be 250 to 900 such genes, comprising 1%–4% of the human genome, depending on the literature source [82–84]. According to the latest findings from the literature, progesterone regulates oligosaccharyltransferase activity. This enzyme switches the N-glycan precursor from the dolichol carrier to the synthesizing protein in the endoplasmic reticulum [69]. At the same time, estrogen is more critical than progesterone for further N-glycan modifications, because it activates the glycosyltransferases B4GalT1 and ST6Gal1, which modify IgG N-glycan ends by the addition of galactose and sialic acid in the Golgi apparatus, respectively [32,85,86]. Testosterone has been associated with glycoprotein sialylation in prostate cancer cells in men [87], but its effect on N-glycosylation in women remains to be investigated. All the results presented in our study regarding estradiol, progesterone, and testosterone demonstrate a potentially relevant role of gonadal sex hormones in mediating IgG N-glycosylation and, most likely, female immunity during the menstrual cycle.
There are also some limitations to our study. For example, since this was the first study on the dynamics of IgG N-glycosylation during the menstrual cycle, we checked for the existence of changes in the IgG N-glycosylation pattern by conducting blood sampling only once a week at equally distributed time points (every 7 days), regardless of menstrual cycle phases, although this was done with a large number of women over a relatively long period of time and thus covered at least two complete menstrual cycles for each woman. Therefore, the measured plasma concentrations of the sex hormones were not a full assessment of the day-today hormone changes during the menstrual cycle. Future studies aiming at a detailed assessment of the specific physiological processes during the menstrual cycle might consider more frequent (daily) blood sampling in order to acquire more precise sex hormone level changes.
《5. Conclusions》
5. Conclusions
To the best of our knowledge, this is the first study that has assessed the dynamics of IgG N-glycosylation in the menstrual cycle. The exact determinants that modulate specific glycan structures on IgG are still not completely elucidated; however, they include already-established genetic and environmental factors, as well as the sex hormonal milieu [88]. IgG glycans are not only important biomarkers but also functional effectors of autoimmune and inflammatory diseases, which are known to be predominant in women and associated with female sex hormones [31]. This study might make it possible to distinguish between differences in physiological menstrual cycle-driven and pathological diseaseassociated changes in the glycosylation of IgG. Moreover, specific IgG glycoforms have been directly linked to many diseases and, within this study, have shown a greater extent of changes during the menstrual cycle within a single person, which might be important in the context of personalized health monitoring by a newly developed glycan biomarker—the GlycanAge index [35]—although this still needs to be explored. However, on the cohort level, changes in IgG glycosylation introduced by the menstrual cycle are small (less than 0.8%), allowing large sample-size studies to sample women regardless of their menstrual cycle phase and to use historical samples when such data is not available. Due to the biological complexity of both the menstrual cycle and the Nglycosylation process, additional hormonal intervention studies are needed to elucidate the mechanistic background linking individual sex hormone concentrations with changes in IgG Nglycosylation.
《Acknowledgments》
Acknowledgments
We are grateful to the medical staff of the Jidong Oilfield Hospital (Beijing, China) for supporting this study. We want to thank Sasa Kralik Oguic from University Hospital Centre Zagreb, Croatia, for her help with measuring sex hormone concentrations. We acknowledge the members of our research groups who carried out daily activities for the project. We are most grateful to our colleague Thomas Klaric for the critical reading of the manuscript and his valuable and constructive suggestions, and our colleague Borna Rapcˇan for help with visualization. Finally, we want to thank the women who participated in the study for their time and efforts.
This research was funded by the European Structural and Investment Funds grant for the Croatian National Centre of Research Excellence in Personalized Healthcare (KK.01.1.1.01), Australia–China International Collaborative Grant (NHMRC APP1112767–NSFC 81561128020), National Natural Science Foundation of China (81773527 and 81573215), and the European Structural and Investment Funds CEKOM (KK.01.2.2.03.0006).
《Authors’ contributions》
Authors’ contributions
Conceptualization: Julija Juric´, Manshu Song, Frano Vucˇkovic´, Marija Pezer, Wei Wang, and Gordan Lauc; methodology: Julija Juric´ and Frano Vucˇkovic´; software: Frano Vucˇkovic´; validation: Julija Juric´; formal analysis: Frano Vucˇkovic´; investigation: Julija Juric´ and Jelena Šimunovic´; resources: Hongli Peng, Manshu Song, Youxin Wang, Jiaonan Liu, Qing Gao, Hao Wang, and Qiaoyun Chu; data curation: Julija Juric´ and Frano Vucˇkovic´; writing—original draft preparation: Julija Juric´, Frano Vucˇkovic´, and Marija Pezer; writing—review and editing: Manshu Song, Irena Trbojevic´ -Akma cˇic´, Wei Wang, and Gordan Lauc; visualization: Frano Vucˇkovic´ and Julija Juric´; supervision: Irena Trbojevic´-Akmacˇic´, Marija Pezer, Manshu Song, Gordan Lauc, and Wei Wang; project administration: Hongli Peng, Manshu Song, Youxin Wang, Marija Pezer, Irena Trbojevic´-Akmacˇic´, Gordan Lauc, and Wei Wang; funding acquisition: Gordan Lauc, Manshu Song, Youxin Wang, and Wei Wang All authors have read and agreed to the published version of the manuscript.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Gordan Lauc is the founder and owner of Genos Glycoscience Research Laboratory, a private research organization specializing in highthroughput glycomic analyses and has several patents in this field; Julija Juric´, Frano Vucˇkovic´, Jelena Šimunovic´, Irena Trbojevic´ -Akmacˇic´, and Marija Pezer are employees of Genos Glycoscience Research Laboratory. The other authors declare that they have no conflict of interest or financial conflicts to disclose.
《Appendix A. Supplementary material》
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eng.2022.10.020.














 京公网安备 11010502051620号
京公网安备 11010502051620号




