《1. Introduction》
1. Introduction
Carbon dioxide (CO2) is the primary greenhouse gas emitted by human activities [1]. According to the Intergovernmental Panel on Climate Change (IPCC) Fifth Assessment Report of Climate Change 2013 (AR5), the atmospheric concentration of CO2 rose to 3.91 × 108 μmol·mol−1 in 2011—more than 40% higher than the pre-industrial level. A significant share of the total CO2 sequestration required for climate change mitigation (about 25% of the total in International Energy Agency (IEA) and IPCC scenarios) is expected to result from carbon capture and storage (CCS). CCS has been intensively investigated in the past couple of decades [2−4]. Potential storage methods include geological storage (storage in oil and gas fields, deep saline formations, or in un-minable coal beds) and ocean storage [5−9]. Current CCS methods are still far from commercialization, due to their high cost and high energy consumption. The storage of CO2 in the subsurface may involve risks such as leakage, contamination of underground water, or even geological disasters [10,11].
CO2 capture and utilization (CCU) methodology is currently more attractive than CCS as the currently available technology for climate change mitigation. Because CCU produces high-value chemical products, it can be used to reduce CO2 emissions with relatively low overall cost and energy expenditure. However, CO2 is considered one of the most stable chemical compounds in the carbon family. Although many publications and much research concern the utilization of CO2 to produce chemicals, mineralization is one of the most efficient methodologies, since it is thermodynamically favorable (Figure 1) [12]. Other methods, such as the conversion of CO2 to fuel and organic chemicals, require more energy than they produce and theoretically generate more CO2.
《Fig. 1》
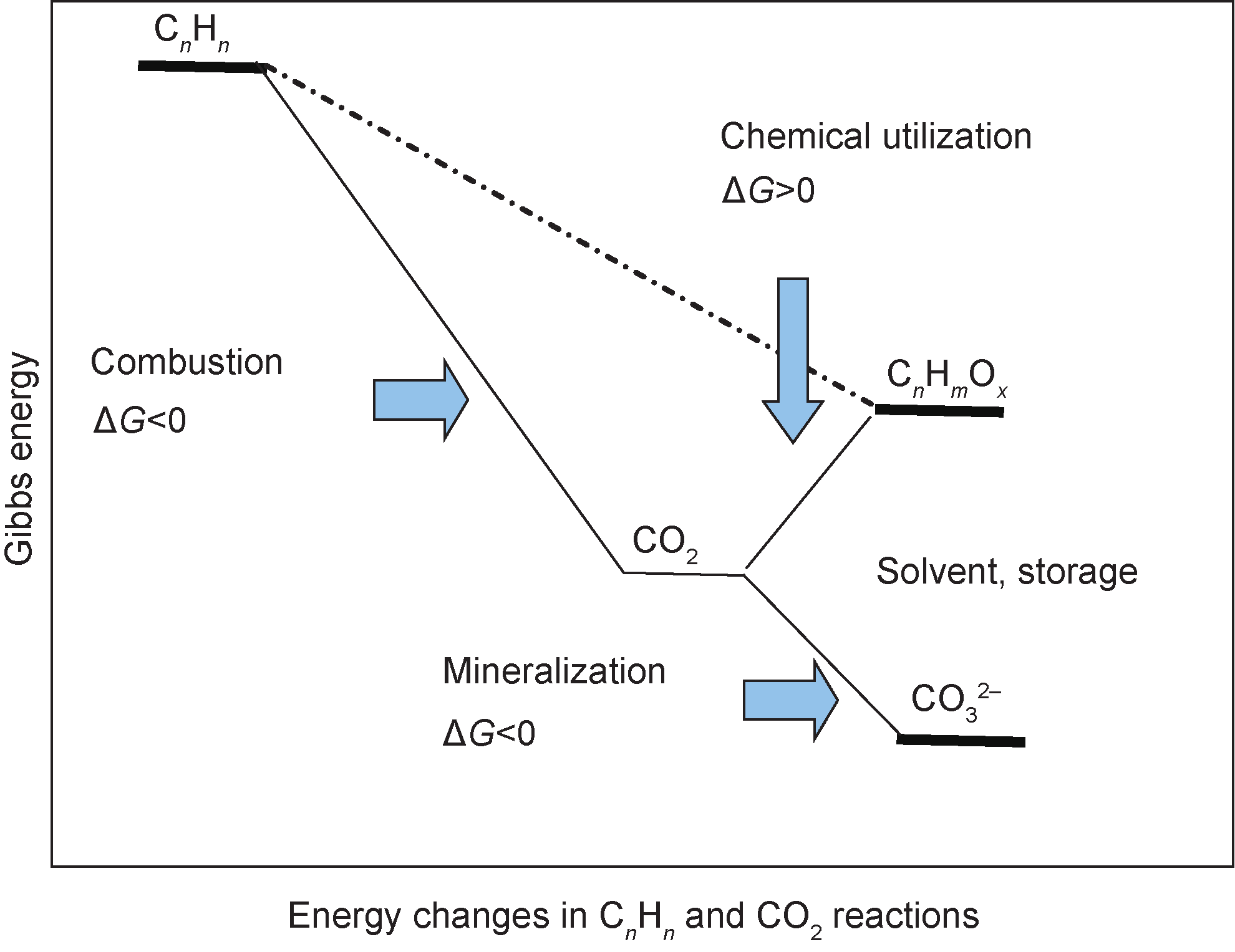
Fig.1 Energy changes in CO2reactions.
CO2 mineralization can store CO2 in a more stable form for thousands of years via reaction with alkaline earth oxides to form carbonates. Some minerals, such as those in volcanic ash and industrial waste, contain basic alkaline earth compounds, which can be reacted with CO2. In recent years, natural minerals such as serpentine and olivine have been extensively investigated for their ability to fix CO2 by mineralization [13], and MgO- or CaO-containing silicate ores have been thermally activated and reacted with CO2. However, activation and mineralization by these methods require a high energy input and have a high cost, and these factors are the major obstacles for the commercialization of these technologies.
Sichuan University has developed a series of technologies to sequestrate and utilize CO2 by mineralization. These technologies have low energy input and are economically viable. Theoretically, the carbonation of CO2 with CaO- or MgO-containing minerals releases energy, which can be recovered. In addition, this process utilizes CO2 to produce valuable chemicals. Energy can be recovered during the mineralization of CO2, and used to produce desired chemicals using their chemical potentials.
Figure 2 shows material fluxes and process steps for CO2 mineralization. This process is coupled with industrial waste conversion, mineral processing, and energy recovery. Phosphogypsum (PG) is a large-scale industrial waste from the production of wet phosphoric acid [14]. The phosphoric acid industry in China deposits about 5 × 107 t of PG every year, resulting in a severe environmental challenge. CO2 mineralization was used to convert the PG to CaCO3 and (NH4)2SO4, which can be used as a construction material and a fertilizer, respectively. Steel slag containing CaO or portlandite (Ca(OH)2) is an industrial waste from steel plants. This material is a toxic deposit due to the leachable CaO. Steel slag is used to produce construction material, and can be used to generate power by CO2mineralization (converting CaO to CaCO3). CO2 mineralization was also applied to potassium extraction from K-feldspar ore. In this process, the K+ ions were first exchanged with the Ca2+ ions and then the Ca2+ ions were fixed by reacting it with CO2 [15]. In addition, an electrolytic method was proposed to intensify the mineralization of CO2 with earth-abundant natural magnesium chloride from salt lakes in order to recover valuable magnesium carbonate. This method can potentially mineralize as much as several billion tons of CO2 [16].
《Fig. 2》

Fig.2 Material fluxes and process steps associated with the CO2 mineralization of natural rocks or industrial residues. (Adapted with permission from Ref. [17] . Copyright 2005 Cambridge University Press)
In this work, we describe several representative CO2-mineralization routes in which natural minerals and industrial waste were used as reaction feedstock/components to produce useful chemicals. We summarize various intensification methodologies, such as the electrochemical method [18], thermal activation [15,19], and the use of additives to enhance the CO2-mineralization reactions [16], with emphasis on scientific progress, engineering applications, and an economic evaluation of these alternative technologies.
《2. CO2 mineralization using industrial waste》
2. CO2 mineralization using industrial waste
《2.1. CO2 mineralization with PG for fertilizer production》
2.1. CO2 mineralization with PG for fertilizer production
PG is a waste deposit from the wet-process phosphoric acid industry. China is the largest manufacturer of phosphorous fertilizer and produces 5 × 107 t PG per year [14]. However, only 15% of this material is used as a set retarder of cement or to make gypsum plaster and bricks [14,20]. The rest—a huge quantity of PG—is mostly discarded in large stockpiles without proper disposal, resulting in land occupation and environmental pollution, particularly to water resources. PG can be converted into a nitrogen fertilizer through the following chemical reaction, which can be used in CCU:
CaSO4⋅2H2O+2NH3+CO2→(NH4)2SO4+CaCO3+H2O (1)
The CO2 contained in flue gas was captured and converted by ammonium-saturated PG slurry to produce (NH4)2SO4 and CaCO3 as per Eq. (1). As mentioned earlier, (NH4)2SO4 is a fertilizer containing both the nutritious elements for plants, nitrogen (N) and sulfur (S), and CaCO3 is a feedstock of cement. This new mineralization approach converted an industrial waste and CO2 into two useful products. It is a thermodynamically favorable process [12]. Figure 3 shows a schematic for the pilot process for CO2 mineralization using PG.
《Fig. 3》

Fig.3 Schematic pilot process for the mineralization of CO2 using PG.
In this process, flue gas was scrubbed with ammonium-saturated PG slurry, and the CO2 contained in the flue gas was captured for conversion into CaCO3 and NH4HCO3. 75% of the CO2 in the scrubber was converted as the flue gas, and the flue gas was scrubbed with acidic PG slurry to reduce the ammonium slip. The ammonium concentration of the exhaust gas was controlled to less than 1.0 × 10−7 μmol·mol−1, which is below the Chinese Emission Controlling Standard (GB 14554—93). The CO2-rich slurry was then pumped into a three-phase reactor, where it was further reacted with fresh PG to form CaCO3 and (NH4)2SO4. The formed CaCO3 in the reactor was filtered, washed, and dried to serve as a building material. Meanwhile, the ((NH4)2SO4 in solution was concentrated in a three-effect evaporation system (see the process flow diagram in Figure 4). (NH4)2SO4 was then crystallized and separated as a fertilizer product.
《Fig. 4》

Fig.4 Illustration of the “one-step”±method for the mineralization of CO2 in flue gas using PG.
A 100 Nm3.h−1 pilot-scale device was successfully operated at the Puguang natural gas purification plant (the pilot device is shown in Figure 5). The main operation parameters were as follows: The flue gas was cooled with circulating water to a temperature of 50 °C and absorbed with ammonia to reduce the concentration of CO2 from 15% to 4.5%. The absorption liquid was mineralized with PG (conversion of 90%) at 75 °C for 6 h to produce CaCO3, which was washed with water, filtered, and then dried using flue gas at 250 °C. Subsequently, the residual solution was evaporated, achieving a 45% concentration of (NH4)2SO4, which was then crystallized to produce a granular (NH4)2SO4 fertilizer. The evaluated income for the conversion per ton of CO2 is about $17 USD [12].
《Fig. 5》

Fig.5 The pilot device at Puguang for the mineralization of flue-gas CO2 using PG.
This process integrated CO2 capture, PG conversion, and fertilizer production. It effectively utilized mineralization as a means of waste processing, and it is both environmental friendly and economically viable. The pilot project has introduced the following technological advances:
(1)The direct capture of CO2 from flue gas using minera-lization;
(2)A new method of producing valuable (NH4)2SO4 ferti-lizer;
(3)The use of industrial waste, PG, as a raw material;
(4)A economical way to integrate CCU as part of a routine process.
《2.2. Mineralization with portlandite for power generation》
2.2. Mineralization with portlandite for power generation
Portlandite is one of the main by-products in industrial alkaline wastes, including carbide slag, steel slag, paper mill waste, kiln dust, and coal fly ashes. Portlandite can easily be reacted with CO2 to yield CaCO3, which is naturally abundant but cheap. On the other hand, sodium bicarbonate (NaHCO3), a rare chemical compound in nature, is an important industrial feedstock for the production of soda ash, baking soda, magnesium carbonate, and so on. With this in mind, NaCl was introduced into this reaction in order to mineralize portlandite to obtain NaHCO3 instead of CaCO3 as the end product. The following carbonation reaction was conceived as a prototype reaction:
Ca(OH)2+2NaCl+2CO2→2NaHCO3+CaCl2(ΔGf0=−62.75kJ⋅mol−1) (2)
The benefit of this reaction is that it uses two industrial pollutants (CO2 and Ca(OH)2) to produce two valuable products (NaHCO3 and energy) concomitantly. This carbonation reaction is theoretically an energy-releasing process, which converts CO2 ( ΔGf0 = −394 kJ.mol−1) into CO32− ( ΔGf0 = −528 kJ.mol−1) [21]. It is essential to use an approach that efficiently harnesses this energy.
Recently, we used reaction (2) to realize an energy-output strategy by harvesting the released energy from the reaction in the form of electricity via a CO2-mineralized fuel-cell (CMFC) system (Figure 6) [22]. This CMFC system consists of a hydrogen-diffusion anode coated with a Pt/C catalyst, a conventional Pt cathode, three chambers separated by an anion-exchange membrane (AEM) and a cation-exchange membrane (CEM), and three buffer tanks to supply the reactants to the individual chambers. The cathode and anode are connected through an external circuit.
《Fig. 6》

Fig.6 Schematic diagram of the CMFC system and the inner structure of a single CMFC. (a) Anode current collector; (b) anode gas chamber frame; (c) hydrogen-diffusion anode; (d) anode liquid chamber frame; (e) AEM; (f) salt chamber frame; (g) CEM; (h) cathode chamber frame; (i) cathode; (j) cathode current collector. (I) Anode buffer tank. (II) Salt buffer tank. (III) Cathode buffer tank. (Reproduced with permission from Ref. [22].Copyright 2014 Science China Press)
The key factor for the generation of electricity from this carbonation reaction is to promote electron transfer, since the acid-base carbonation reaction entails no electron-transfer process, which is required to generate electricity directly. Because the electricity can be generated in an opposite direction, we used membrane electrolysis to produce acids and bases from salts using electricity. The reversed electrolysis process in the CO2-mineralized fuel cell was chosen to enhance the CO2utilization.
In this system, the highly valuable product, industrial NaHCO3, was produced concomitantly during electricity generation. The highest power density of this system was 5.5 W.m−2, higher than that of many microbial fuel cells. The maximum open-circuit voltage was 0.452 V. Moreover, this system was demonstrated to be viable for low concentrations of CO2(10%) and for other carbonation processes. Therefore, this new strategy is an energy-generating and environmentally friendly approach to utilize CO2, and could be used as a supplement to current CO2-emission scenarios.
《3. CO2 mineralization using natural minerals》
3. CO2 mineralization using natural minerals
《3.1. Electrolytic CO2 mineralization with MgCl2 to produce MgCO3》
3.1. Electrolytic CO2 mineralization with MgCl2 to produce MgCO3
Magnesium chloride (MgCl2) is an abundant natural resource that is widely distributed in seawater, salt lakes, and ore minerals. The average concentration of magnesium ions in seawater is about 0.13%, which can potentially mineralize as much as 3.34 × 109 t of CO2 (111 years’ worth of global CO2 emissions). The prospective reserves of magnesium chloride salt in the four major saline lakes in China are estimated to be as high as several billion tons.
An electrolytic method was proposed to intensify the mineralization reaction of flue-gas CO2 with magnesium chloride (0.1 mol.L−1) to recover valuable magnesium carbonate (MgCO3) (Figure 7) [18]. First, magnesium chloride was converted into magnesium hydroxide and hydrochloric acid by electrolysis. Next, the resulting magnesium hydroxide was reacted with CO2 to produce a magnesium bicarbonate solution. The magnesium carbonate product was finally obtained by calcination of the Mg(HCO3)2.
《Fig. 7》

Fig.7 Illustration of an electrolysis cell and the process for the conversion of magnesium chloride to magnesium carbonate. (Reproduced with permission from Ref. [18]. Copyright 2014 Springer)
In the electrolysis cell, H2 is oxidized to form H+ ions with the help of a special nickel-foil anode. The H+ ions react with the Cl− ions diffusing through the AEM membrane from the cathode side of the cell to form HCl. Water is electrolyzed on the cathode and transformed into H2, which is recycled to the anode. CO2 mineralization occurs on the cathode side, where the Mg(OH)2 reacts with CO2 to form MgCO3.
Both pure and diluted CO2 gas can be used directly in this mineralization process. CO2 concentration can be reduced to below 20%, which is a normal concentration for industrial flue gas. This mineralization method avoids the high-energy-consuming process of CO2 purification and greatly reduces the energy consumption of CO2 mineralization. About 871 kWh (about $70 USD according to the price of electricity on March 16, 2015) of electricity at a cell voltage of 0.7 V is required to mineralize 1 t of CO2 and produce 3.16 t of MgCO3. According to the market price of magnesium carbonate ($480 USD.t−1, according to the price from the CHEMinfo website http://www.cheminfo.cn/) on March 10, 2015), CO2 mineralization by means of magnesium chloride is an efficient (net effect of 50%−70%) and economical way to reduce CO2 emissions.
《3.2. CO2 mineralization with natural K-feldspar to produce potash fertilizer》
3.2. CO2 mineralization with natural K-feldspar to produce potash fertilizer
Soluble potassium resources are scarce in many parts of the world (<1% of global potassium storage), including China. On the other hand, K-feldspar (KAlSi3O8) is an abundant and stable insoluble potash ore with reserves of more than 1.0 × 1010 t; therefore, the production of potash fertilizer from K-feldspar is crucial in order to reduce the consumption of soluble potassium resources [23].
3.2.1. K-feldspar-phosphogypsum co-activation and mineralization process
A coupling process (Figure 8) comprising the activation of K-feldspar with PG at high temperature was investigated as a method of extracting K2SO4 and mineralizing CO2. The main reactions in this activation and mineralization process are:
2KAlSi3O8+CaSO4=K2SO4+CaAl2Si2O8+4SiO2 (3)
CaAl2Si2O8+CO2+2H2O=CaCO+Al2Si2O5(OH)4 (4)
This process extracts potassium from natural K-feldspar ore and simultaneously deals with PG waste [15]. The K-extraction and CO2-mineralization rates were significantly affected by activation parameters. Under specific activating conditions—1200 °C for 2 h with an ore/CaSO4 mass ratio of 1 : 2, and mineralizing at 100 °C with an initial CO2 pressure of 4 MPa—the K-extraction and CO2-mineralization yields exceeded 87% and 7.7%, respectively.
《Fig. 8》
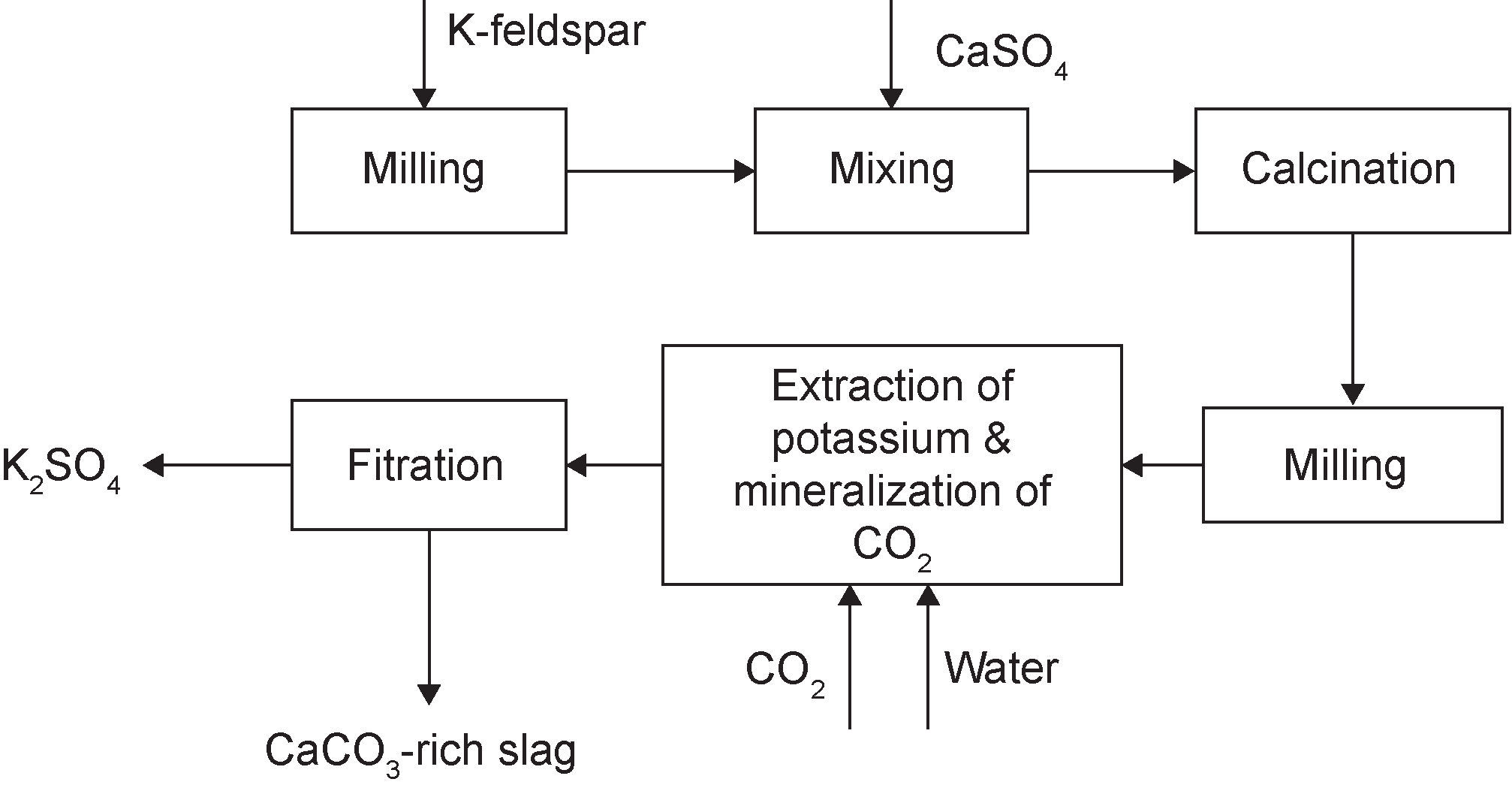
Fig.8 Schematic diagram of the thermal activation of K-feldspar and PG for CO2 mineralization. (Reproduced with permission from Ref. [15]. Copyright 2014 American Chemical Society)
This potassium-extraction process follows an ion exchange reaction of Ca2+ from CaSO4 with K+ from K- feldspar, in which CO2 reacts with CaAl2Si2O8. The K+ions exchange with Ca2+ ions to form K2SO4, and the remaining molecular skeleton after this ion exchange forms an electrical neutral phase CaAl2Si2O8. The CaAl2Si2O8 is then dissolved by H+, which results from the ionization of H2CO3, and Ca2+ is released. Finally, the Ca2+ is reacted with HCO3− to form CaCO3 [24].
The reaction activation temperature of 1200 °C is much lower than that of the decomposition temperature of K-feldspar (1500 °C), which significantly reduces the energy consumption of this process. The reaction temperature is expected to be in the range between the particle surface melting (half-melting) and the melting point, in order to maintain the fluidity of the reactants in commercial production. The co-activation of K-feldspar with PG facilitates the formation of a molten phase in the calcination reaction, which enhances the mass transfer and reaction rate and reduces the reaction temperature. Therefore, this process is a viable alternative approach for the application of natural K-feldspar and industrial solid waste, with a relatively low energy consumption.
To evaluate the economic efficiency of this co-activation and mineralization process, the primary balance of the materials and energy needed for the mineralization of one ton of CO2 was calculated based on the experimental data (Figure 9, y denotes the amount of CO2 mineralized by K-feldspar). The results showed that the primary cost is the input of ore and the energy consumption, while the primary income is the production of K2SO4. According to the CHEMinfo website, the present market value of K2SO4 is about $600 USD.t−1, so the net profit for this mineralization technology is $200 USD.t−1 of CO2. Therefore, this coupled activation and mineralization process is an economically feasible method to reduce CO2 emissions.
《Fig. 9》
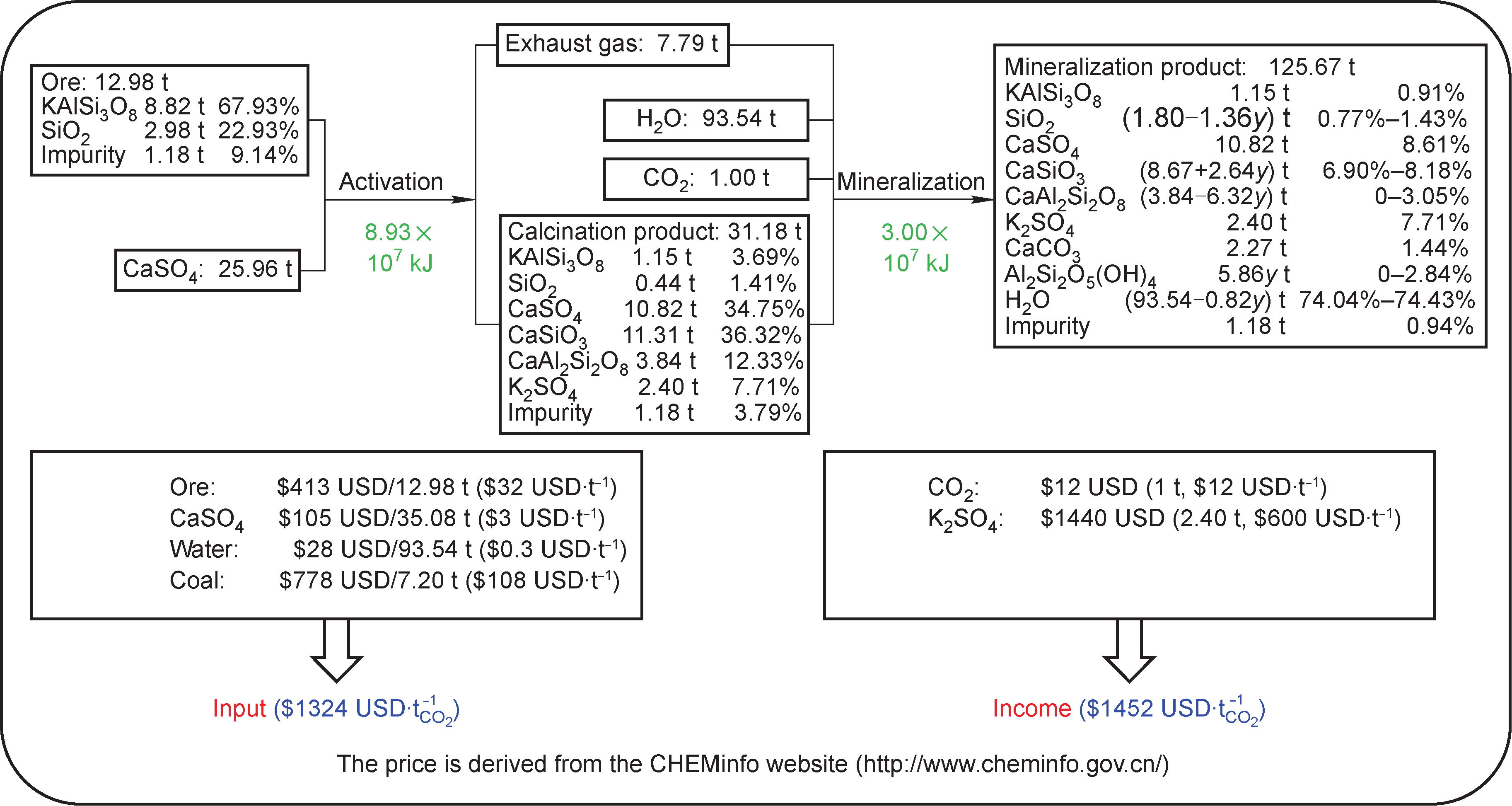
Fig.9 An economic analysis of the K-feldspar-phosphogypsum co-activation and mineralization process, based on the mineralization of 1 t of CO2.
3.2.2. K-feldspar-CaCl2 co-activation and mineralization process
In order to further reduce the activation temperature and the energy consumption, a compound with a lower melting point, CaCl2 (ca. 782 °C), was used as the promoter for the thermal activation of K-feldspar and mineralization of CO2 to extract soluble potassium [19]. CaCl2 is an industrial waste with limited practical use, deposited by the Solvay process of soda-ash production. Therefore, this new process is environmentally friendly, due to its consumption of the CaCl2 waste.
Activation temperature, reaction time, and the K-feldspar/CaCl2 ratio are the important factors in this K-extraction and CO2-mineralization system. A fairly high K-extraction ratio (over 90%) and CO2-mineralization ratio ( 0.12gCO2/gK-feldspar) were obtained at an activation temperature of 908.3 °C−a much lower temperature than that of the PG co-activation system. As shown in the reaction model (Figure 10) the use of an appropriate temperature and CaCl2 content facilitates the exchange of Ca2+ with K+ and the formation of CaAl2Si2O8, Ca3Si3O9, and CaSiO3, due to the hydrolysis of CaCl2 and the collapse of the K-feldspar framework. CO2 is fixed by reactions with Ca3Si3O9 and CaSiO3, resulting in the mineralization product, CaCO3. The results also showed that the pH of the solution was a significant factor for CO2 mineralization. Low pH accelerates the leach of Ca2+ but restrains the absorption of CO2, while high pH increases the concentration of CO32−. Therefore, an appropriate pH, CO32− concentration, and Ca2+ concentration are key factors for the precipitation of CaCO3 in this mineralization reaction.
《Fig. 10》
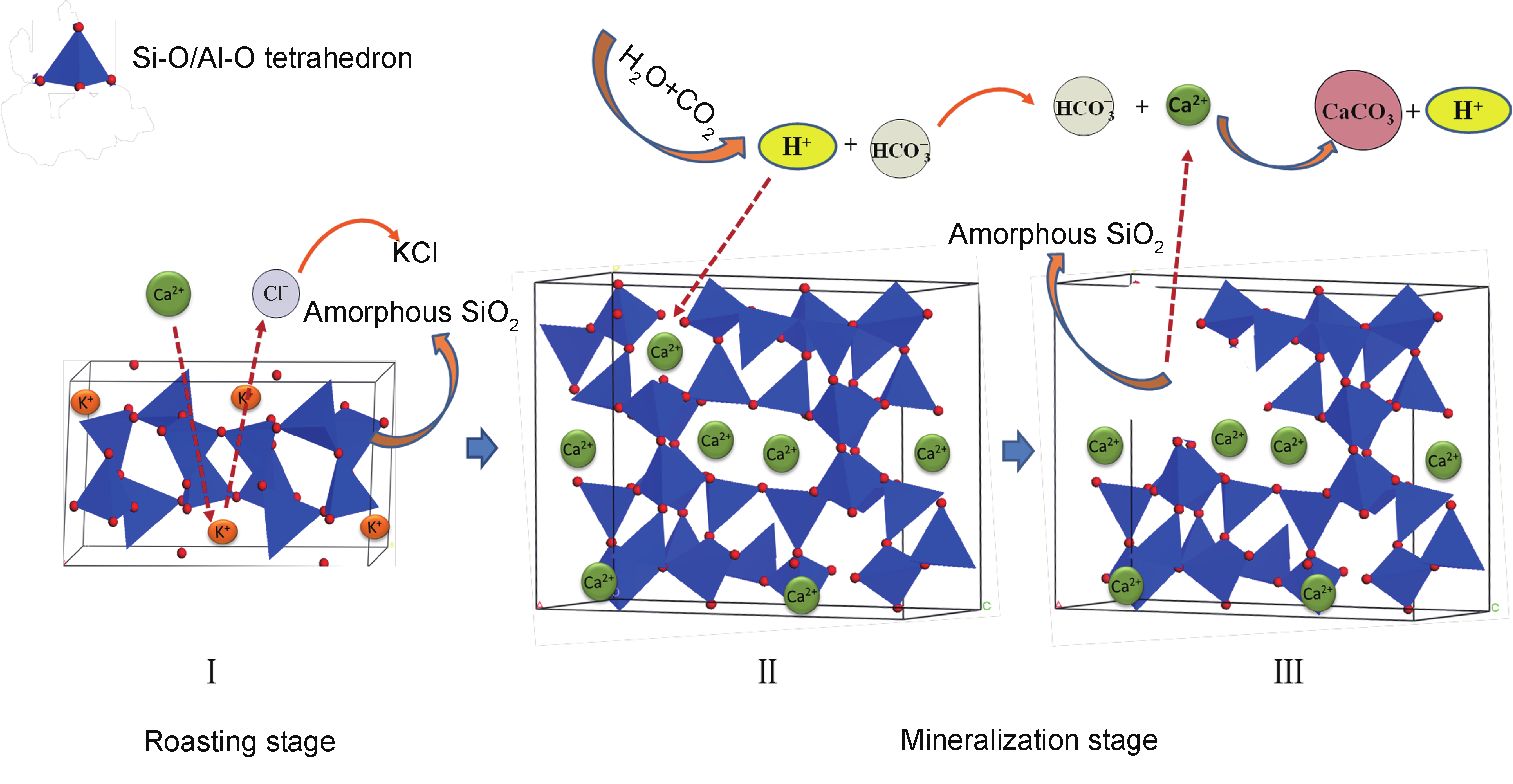
Fig.10 Schematic diagram of the mechanism of the activation and mineralization stage. (Reproduced with permission from Ref. [19]. Copyright 2014 American Chemical Society)
The results indicate that this alternative technology utilizing insoluble K-feldspar, CaCl2 industrial waste, and CO2 mineralization is a promising process with the potential to produce potash fertilizer and reduce CO2 emissions.
3.2.3. The technological process for mineralization of 500 t.a−1 CO2 with K-feldspar
To evaluate the potential of this mineralization technology for industrial application, a scale-up pilot plant was designed on the basis of the experimental results with a mineralization ability of 500 t of CO2 per year. Figure 11 shows the principal process.
《Fig. 11》
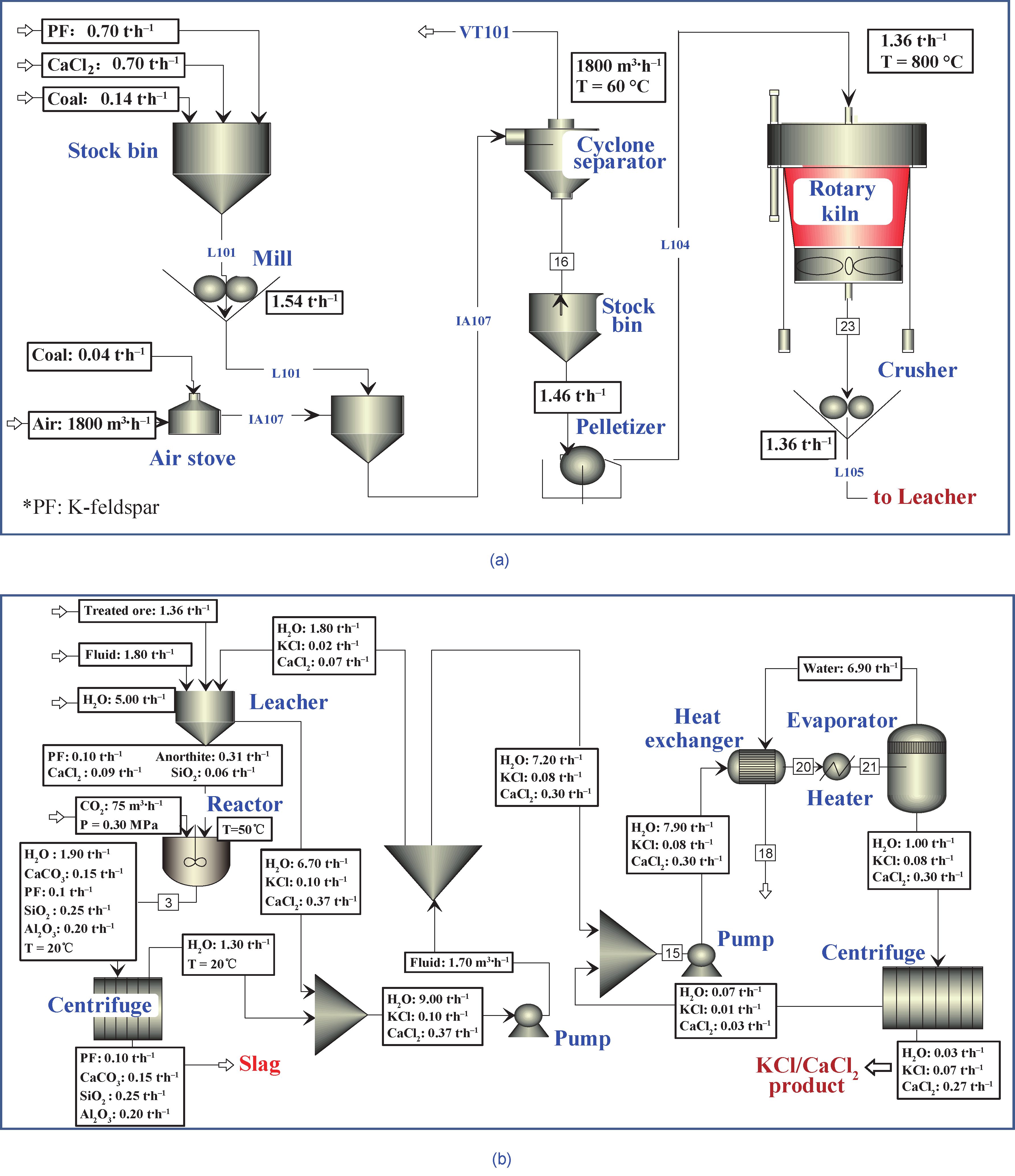
Fig.11 The technological process for mineralization of 500 t.a−1 CO2 with K-feldspar.(a) Pretreatment and activation section; (b) leaching and mineralization section.
This process involves four main steps: pretreatment, activation, leaching, and mineralization. K-feldspar, CaCl2, and coal were first mixed and milled to a particle size below 150 µm, and then shaped and activated at an appropriate temperature (e.g., 800−900 °C) in order to exchange K+ with Ca2+ ions from anhydrous calcium chloride. The activated slag was leached with hot deionized water (at a temperature of ca. 80 °C) to extract potassium, and then CO2 was bubbled in the suspension of the residue at 0.3 MPa and 50 °C for 2 h, for mineralization of CO2 to occur. The slag was centrifuged and filtered after mineralization, and the filtrate and washing solutions were concentrated to obtain the KCl and CaCl2 products. This pilot plant has been designed and is currently under construction. At this plant, the technological parameters of the process will be investigated systematically, to develop further understanding and to pave the way for industrial applications.
《4. Conclusions and future scope》
4. Conclusions and future scope
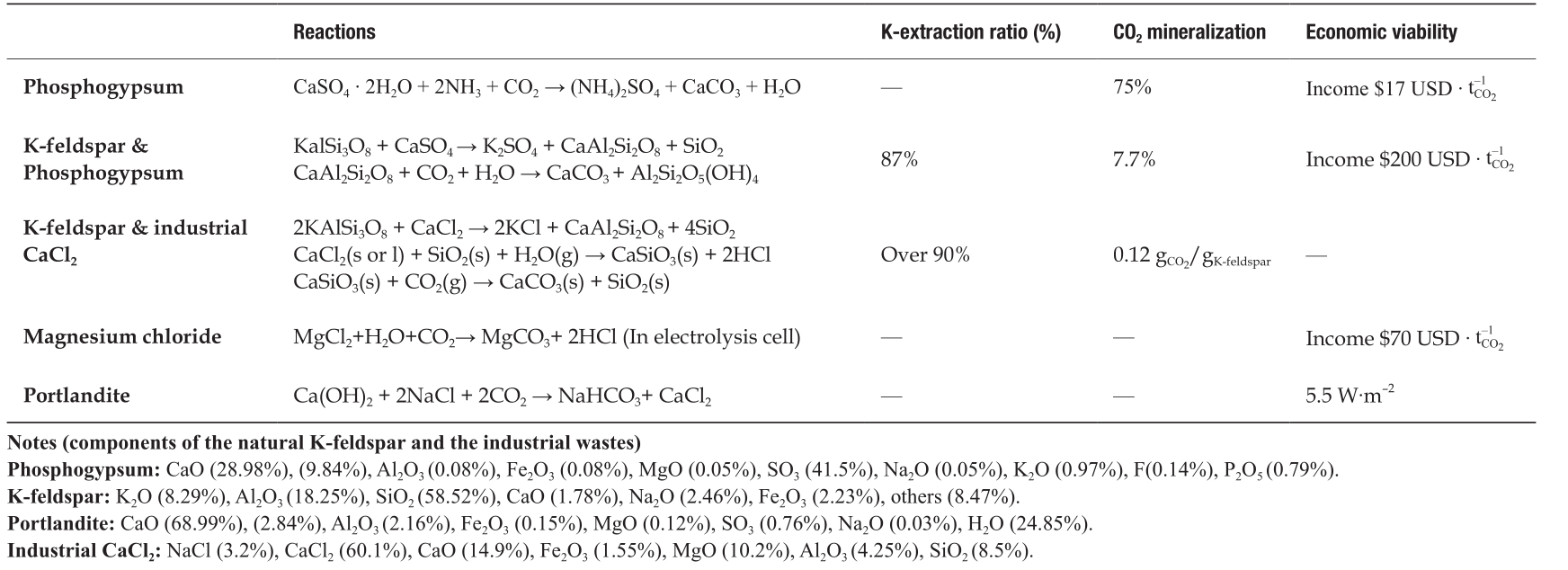
In recent years, Sichuan University has made remarkable progress in the development of CO2-mineralization technologies with high efficiency, low cost, and low energy consumption. We summarize the reactions, relative reactivity, and economic viability for the representative CO2–mineralization technologies in Table 1. Based on the cost analysis of these performed processes, the presented CCU techniques exhibit excellent economic viability and potential for application. In this context, we anticipate that research in CO2 mineralization will become even more widespread in the near future, particularly for the coupled technologies of natural resource exploitation and industrial waste treatment. The technologies described in this paper have the potential to process large quantities of CO2 and industrial waste, while producing high-value chemicals.
《Tab.1》
Tab.1 Comparation of the reactions, relative reactivity and the economic viability for the processes.
CO2 mineralization using natural minerals and industrial wastes has recognized and well-documented advantages, such as very large capacity, no requirement for post-storage monitoring, and overall exothermic processes. However, a number of significant challenges still exist, and must be faced and addressed. Potential exists for breakthroughs in the development of more efficient technologies and optimal systems that allow for the practical mineralization and utilization of CO2. Future research should be encouraged in the following areas.
(1)At present, these processes are still somewhat expensive and are not fully economically favorable. Efforts should be made to assess large-scale technical feasibility and corresponding energy requirements, and to develop simple and cheap methodologies for CO2 storage.
(2)Expanding the advertisement to increase public understanding and support is crucial. Public acceptance might be enhanced by the fact that this storage method is highly verifiable and unquestionably permanent.
(3)Integration of power generation, mining, the carbonation reaction, the carbonates’ disposal, and the associated transport of materials must occur to enable energy needs to be optimized in a site-specific manner.
(4)Future work in mineral carbonation requires the development of a series of demonstration plants in order to enable a deeper understanding of the mineralization processes for industrial applications.
《Acknowledgements》
Acknowledgements
The authors are grateful for the finance support of the Ministry of Science and Technology (State Key Research Plan, 2013BAC12B00) and the National Natural Science Foundation of China (21336004 and 51254002).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Heping Xie, Hairong Yue, Jiahua Zhu, Bin Liang, Chun Li, Yufei Wang, Lingzhi Xie, and Xiangge Zhou declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




