《1. Introduction》
The invention and development of new research concepts, novel methodologies, and novel bioanalytical techniques are essential in advancing the animal sciences, which include feed and nutrition science [1]. Common and conventional wet chemistry methods are often used for nutritional analysis and feed evaluation. However, wet chemistry methods usually destroy the inherent molecular structure of the feed during preparation for lab digestion and analysis processing [1–3] because these wet analytical methods include heavy applications of harsh chemicals. These chemicals often destroy or alter the native or original inherent molecular structure of the feed, and often generate artifacts that affect feed and nutrition evaluation [1–2].
Recently developed advanced synchrotron radiation infrared microspectroscopy (SR-IMS) is a fast, noninvasive, and direct bioanalytical technology [4–7]. This cutting-edge bioanalytical technology has brilliant light (a million times brighter than sunlight), nondivergent beam light, and an effective small source size [5,6]. It is capable of revealing the molecular structure of biological tissue at ultra-high spatial resolutions [4,8–12]. Using synchrotron-based bioanalytical technology makes it possible to obtain several types of information simultaneously (Fig. 1): tissue structure, tissue nutrition, tissue chemistry, and tissue environment [2,13,14].
《Fig. 1》

Fig. 1. Advanced synchrotron-based bioanalytical technology can provide four kinds of information simultaneously, including tissue structure, tissue nutrition, tissue chemistry, and tissue environment.
To date, little application has been found in the animal science community for the use of SR-IMS to study the interactive relationship between feed molecular structure and molecular nutrition or conventional animal nutrition. Similarly, little application has been found for the use of advanced synchrotron-based bioanalytical technology to explore the inherent structural makeup of the cellular and subcellular dimensions in animal feeds, which are associated with nutrient delivery in animals [15,16]. Along with other factors such as the nutrient matrix, the inherent molecular structure and makeup of a feed affects feed quality, nutritive value, biofunction, fermentation behavior, degradation kinetics, and digestion in animals [12,17]. The feed molecular structure conformation or structural makeup strongly impacts the protein that is absorbed in the small intestine, and strongly impacts the protein’s accessibility to internal digestive enzymes in the animal gastrointestinal tract [18,19]. Reduced accessibility to internal digestive enzymes causes poor digestion and poor absorption, and thus results in poor protein nutritive value in animals [20–22].
The objective of this article is to introduce our novel research ideas and bioanalytical techniques (i.e., the advanced synchrotronbased bioanalytical technology) as a new approach for the animal science community in quantifying the interactive relationship between feed molecular nutrition and molecular structure.
This review article covers the following material. Section 2 presents the concept of synchrotron-based bioanalytical technology, along with the major components of this technology. It then presents some synchrotron molecular spectroscopy techniques. Section 3 presents applications of this technology in the form of advanced synchrotronbased research programs. Section 4 follows with conclusions.
《2.Nutrition and feed research programs based on novel synchrotron-based bioanalytical technology》
2.Nutrition and feed research programs based on novel synchrotron-based bioanalytical technology
《2.1. The concept of a synchrotron radiation facility》
2.1. The concept of a synchrotron radiation facility
What is a synchrotron? A simple answer is that a synchrotron is a giant particle accelerator that turns electrons into light [4–6]. A synchrotron radiation facility includes various components such as an electron gun, a linear accelerator, a booster ring, a storage ring, many beamlines (e.g., an infrared line, soft X-ray line, and hard X-ray line), and experimental hutches or stations [4–6]. A mid-sized synchrotron radiation facility is roughly the size of a football field; one example of a mid-sized facility is the Canadian National Synchrotron Radiation Facility—Canadian Light Source (CLS), which is located at the University of Saskatchewan in Saskatoon, Saskatchewan, Canada. However, some synchrotron radiation facilities are larger, such as the Advanced Photon Source in Chicago, Illinois, USA; the National Synchrotron Light Source II in Upton, New York, USA; and the SPring-8 in Harima Science Park City, Hyogo Prefecture, Japan. The size of the facility is partially dependent on the synchrotron target energy levels (ranging from 0.8 GeV to 8.0 GeV).
《2.2. Using a synchrotron radiation facility for feed molecular structure research》
2.2. Using a synchrotron radiation facility for feed molecular structure research
Synchrotron light is extremely brilliant; it is a full-spectrum photon beam and a source of electromagnetic radiation. The accelerator causes electrons to move at rapid speeds with high energy. Bending magnets and device undulators (or “wigglers”) in the synchrotron facilities transform the high-energy electron beam into a photon beam. This photon beam is called “synchrotron light.” Scientists usually work at experimental stations at the end of each synchrotron beamline to study molecular structure though an analysis of the synchrotron-based spectrum [1,5,6,23,24]. The only disadvantage of using this technology is the necessity of having access to a synchrotron facility, which costs millions of dollars to build.
《2.3. Plant-based amides research using cutting-edge synchrotron-based bioanalytical technology》
2.3. Plant-based amides research using cutting-edge synchrotron-based bioanalytical technology
Plant-based feeds, seeds, green forage, and silage protein have unique molecular chemical makeups or molecular conformations; therefore, the molecular spectrum for each plant-based feed protein is unique. The spectrum of a plant-based feed protein in the vibrational middle-infrared region contains two important and significant characteristics: the protein amide I spectrum, with a spectral peak at about 1600–1700 cm–1, and the protein amide II spectrum, with a spectral peak at about 1500–1560 cm–1. These two unique spectral peaks are due to protein backbone vibrations—that is, stretching and bending [25–28]. The plant-based feed protein amide I spectrum, rather than the protein amide II spectrum, is usually used for protein α-helix, protein β-sheet, protein random coil, and protein β-turn analysis [29,30].
《2.4. Multivariate molecular spectral analyses using cutting-edge synchrotron-based bioanalytical technology》
2.4. Multivariate molecular spectral analyses using cutting-edge synchrotron-based bioanalytical technology
To detect differences in the molecular structure of plant-based feeds, multivariate techniques or methods can be used to analyze the molecular spectra from feeds. Two of these methods are agglomerative hierarchical molecular spectral cluster analysis and principle component analysis. In these multivariate analyses, it is not required that the spectral assignments be known, because the aim is simply to discriminate between and qualitatively separate treatments that impact molecular structure and induce molecular structure changes that may affect nutrient absorption in animals [30–33].
《3.Applications and studies using synchrotron-based bioanalytical technologies for feed and molecular nutrition research》
3.Applications and studies using synchrotron-based bioanalytical technologies for feed and molecular nutrition research
《3.1. Application I: Molecular chemistry imaging of animal feeds》
3.1. Application I: Molecular chemistry imaging of animal feeds
The first application involves using synchrotron-based bioanalytical technologies for the molecular chemistry imaging of animal feeds [31]. Examples of this application include imaging the molecular chemistry of wheat [4], Pioneer corn [34], and sorghum [13]. These studies were carried out by our team at the National Synchrotron Light Source at Brookhaven National Laboratory (NSLS-BNL, US Department of Energy) or at CLS (University of Saskatchewan) in order to examine the effect of processing treatment on the cotyledon tissues in yellow types of Brassica canola seeds grown in Western Canada [14]. Using feed molecular imaging, it is also possible to see the differences between frostor freeze-damaged cereal grain seeds and normal seeds (e.g., for wheat).
《3.2. Application II: Detection of molecular structural changes in plant-based feed induced by gene transformation and gene modification》
3.2. Application II: Detection of molecular structural changes in plant-based feed induced by gene transformation and gene modification
The second application uses synchrotron-based bioanalytical technologies to detect foreign structural changes in protein makeup or conformation that were induced by gene transformation, gene inserting or gene silencing. This research was carried out by our team at the NSLS-BNL and the Advanced Light Source at Lawrence Berkeley National Laboratory (ALS-LBNL). Our team performed studies that applied cutting-edge synchrotron techniques to compare and distinguish differences in the molecular structures of control alfalfa protein (i.e., with no foreign gene inserting) and transgenic alfalfa plant tissue (in which the foreign maize Lc regulatory gene was inserted at subcellular levels). It also quantified the structural conformation in the protein biopolymer using multicomponent peak modeling with Gauss-Lorentz methods [35–38]. At present, our team is applying synchrotron-based bioanalytical technology to study the impact on alfalfa molecular structure of inserting a double gene and two foreign genes [39,40], and to explore the impact of gene silencing on the structural changes in the alfalfa [41]. All these structural studies are linked to nutrient delivery studies. Our results showed that single Lc gene transformation produced 2 kg more milk per day per cow, for a 650 kg cow grazing only on alfalfa pasture with a dry matter intake of 17 kg [36–38].
《3.3. Application III: Detection of heat-induced protein structural modification in feed at a molecular level》
3.3. Application III: Detection of heat-induced protein structural modification in feed at a molecular level
The third application involves using synchrotron-based bioanalytical technologies to detect heat-induced protein structural and sub-fraction features that affect rumen degradation and the intestinal digestion of protein in ruminant animals [9,10,42,43]. Several studies were carried out by our team using advanced synchrotron technology as a novel tool with a novel approach. These studies revealed the internal structures of feed tissues that were affected by various treatments, and quantified the interactive relationship between protein structure and nutrition [9,10,19,41–45]. With synchrotron technology as an advanced tool, it is possible to study not only the inherent molecular structure of protein biopolymers, but also the molecular structure of carbohydrate biopolymers [17] and the structural makeup of lipid biopolymers. In the formulation of a ruminant diet, the total metabolizable protein and the degraded protein balance are the two most important parameters. With this novel tool, it is possible to develop a prediction equation based on the molecular structure features of the feed in order to predict these two parameters without conducting time-consuming, expensive, and metabolic trials with dairy cows (Fig. 2).
《Fig. 2》

Fig. 2. It is a time-consuming and expensive process to determine the metabolizable protein of a feed or diet. CP: crude protein; CHO: carbohydrate; GE: gain energy; kp: rate of passage; kd: rate of degradation; RDC: rumen degradable carbohydrate; RDP: rumen degradable protein; OEB: degraded protein balance; MCP: microbial crude protein; AMCP: truly absorbed microbial protein in the small intestine; ARUP: truly absorbed rumen undegraded protein in the small intestine; DVE: truly digested protein in the small intestine; NE: net energy; UCP: undigestable crude protein; ENDP: endogenous protein in the small intestine; FPCM: fat-protein-corrected milk; UOM: undigestible organic matter. (Adapted from our team member Arjan Jonker)
《3.4. Application IV: A study of the impact of bio-ethanol processing on the inherent molecular structure of feed》
3.4. Application IV: A study of the impact of bio-ethanol processing on the inherent molecular structure of feed
The fourth application uses synchrotron-based bioanalytical technologies to detect changes in molecular structure resulting from bioenergy/biofuel processing. Our team [16] also examined the interactive relationship between the molecular structure of protein biopolymers and the metabolic characteristics of protein in animals. The study was conducted to reveal molecular makeup and conformation in protein that was co-produced during bioenergy production, and to distinguish the differences between the original feedstocks and various co-products [16]. Our team is currently using this advanced technique to reveal new co-products (i.e., carinata meal) of bioenergy processing in comparison with traditional co-products (i.e., canola meal) of bio-oil processing, for use as a new feed source for dairy cows and beef cattle [45,47].
《4.Summary and implications》
4.Summary and implications
In conclusion (Fig. 3), the studies carried out by our team, as discussed in this review, provide a new concept for advanced feed and nutrition research on a molecular basis. They demonstrate the potential of synchrotron-based techniques for revealing inherent molecular structural conformation changes in livestock feed at ultra-high spatial resolution after different types of treatments and processing. This cutting-edge technique can be used to reveal the interactive relationship between changes in molecular structure and nutrient absorption in both ruminant and monogastric animals.
《Fig. 3》
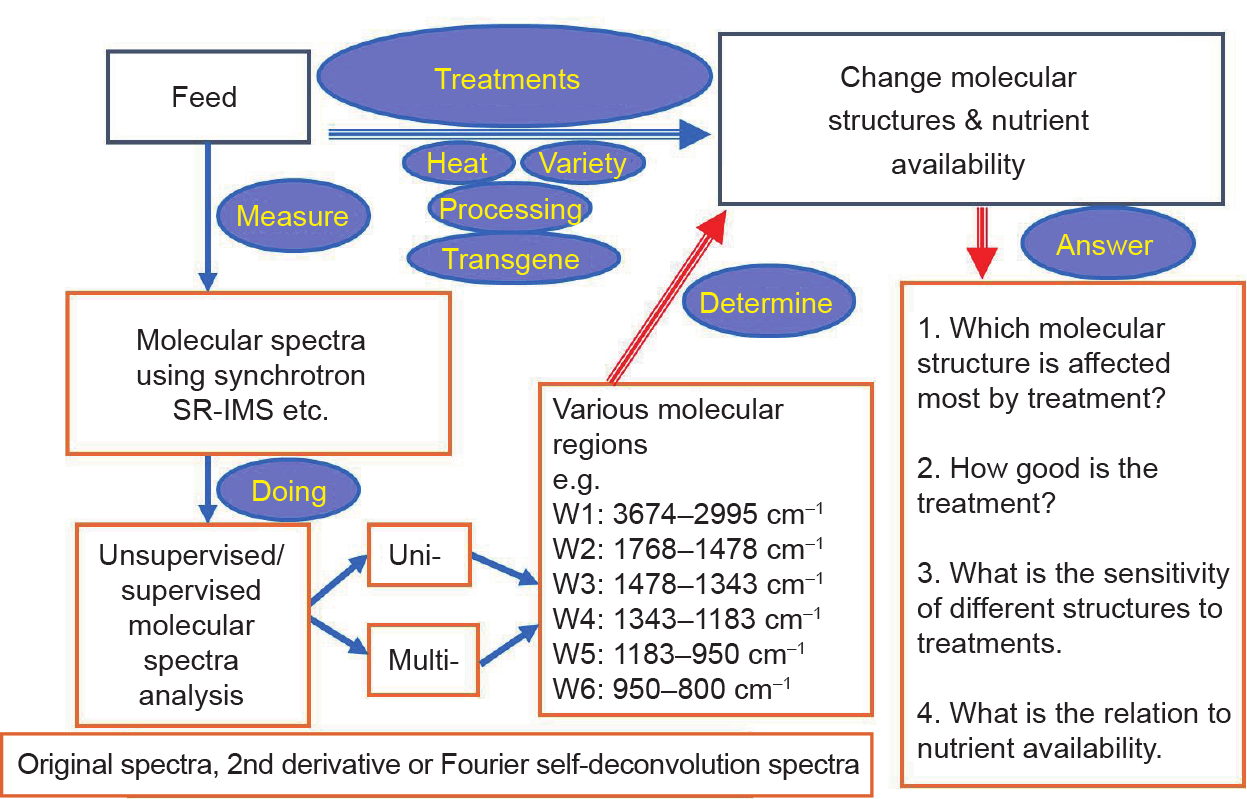
Fig. 3. Summary and implications of a synchrotron-based molecular spectroscopic approach.
《Acknowledgements》
Acknowledgements
The National Synchrotron Light Source in Brookhaven National Laboratory (NSLS-BNL, New York, USA) and the Advanced Light Source in Lawrence Berkeley National Laboratory (ALS-LBNL) are supported by the US Department of Energy. Canadian Light Source Incorporated at the University of Saskatchewan (Saskatoon, Canada) is supported by various Canadian federal and provincial funds. The authors are grateful to Drs. Lisa Miller (NSLS-BNL), Chithra Karunakaran, Tim May (Canadian Light Source), Hans Bechtel (ALS-LBNL, Berkeley), etc. for synchrotron beam time, discussion and/or collaborations, to Randy Smith at NSLS-BNL (New York) for helpful synchrotron data collection, and to Ferenc Borondics, Xia Liu, Tor Pederson, Luca Quaroni, etc. for helpful data collection at the 01B1-1 station, Canadian Light Source. Feed Research Chair Programs have been supported by the Natural Sciences and Engineering Research Council of Canada (NSERC-Individual Discovery Grant and CRD Grant), the Ministry of Agriculture Strategic Feed Research Chair Program, the Saskatchewan Agriculture Development Fund, Saskatchewan Canola Development Commission, SaskPulse Growers, Western Grain Foundation, the Saskatchewan Forage Council, etc. The authors thank our previous team member Dr. Arjan Jonker (Research Scientist, Grasslands Research Center, New Zealand) for adapting his figure in this invited review article.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Peiqiang Yu and Luciana L. Prates declare that they have no conflict of interest or financial conflicts to disclose.













 京公网安备 11010502051620号
京公网安备 11010502051620号




