《1.Introduction》
1.Introduction
Protein restriction is typically recommended for patients with kidney and liver diseases. In farm animals, low-protein diets are widely accepted because of limited feed resources and high feed costs. However, low protein intake causes a decrease in the protein deposition of most tissues in humans and animals, which is not beneficial for patients’ health [1] or animal growth [2]. Protein malnutrition can cause a diminution of muscle size, which may be partly attributed to limited substrate availability for protein synthesis in muscles [2–4].
As one of 10 essential amino acids (EAAs), leucine plays an important role in protein synthesis and degradation in humans and animals, and specifically in the postcibal promotion of protein synthesis and suppression of protein decomposition in muscles [5]. The beneficial effects of leucine in protein synthesis occur through the mammalian target of rapamycin (mTOR) signaling pathway, and it has been suggested that both the eukaryotic initiation factor 4E binding protein 1 (4E-BP1) and ribosomal protein S6 kinase 1 (S6K1) can be increasingly phosphorylated [6–8]. It was demonstrated that a chronically protein-restricted diet suppresses the mTOR signaling pathway and thus decreases the rate of protein synthesis [2,9]. Therefore, we hypothesized that leucine supplementation in a severely protein-restricted diet could stimulate skeletal muscle protein synthesis in rats, and then maintain the muscle mass.
The aim of this study is to elucidate the effect of the supplementation of leucine to a greatly protein-restricted diet on the weight and protein synthesis of skeletal muscle in a rat model, and to examine the mechanisms involved. Since it has been demonstrated that oral administration of leucine can enhance the phosphorylation of both 4E-BP1 and S6K1 in rats [10], we examined the phosphorylation of these two factors to investigate whether leucine worked in the same way.
《2.Materials and methods》
2.Materials and methods
《2.1. Animals and diets》
2.1. Animals and diets
All animal facilities and experimental procedures used in this study were reviewed and approved by the China Agricultural University Animal Care Committee (Beijing, China). Thirty-six male Sprague-Dawley rats (200 ± 2.7 g, 13 weeks old) individually received the AIN93-G diet, prepared according to the American Institute of Nutrition Guidelines, ad libitum for 5 d to allow adaptation to conditions. The rats were kept at 24 °C with 12 h light and had free access to drinking water. At the end of the adaptation period, we divided the rats into one of three dietary treatments according to their body weight. The experimental diets were as follows: the standard diet in AIN93-G with 20% casein diet (normal protein, CON); a 10% casein + 0.44% alanine diet (protein restricted, R); and a 10% casein +0.87% leucine diet (protein restricted, RL). Leucine was supplemented in the RL diet to make the leucine content equal to that in the CON diet. Alanine was added to the R diet to obtain a diet that was isonitrogenous to the RL treatment. The same amount of all EAAs, except for leucine, was added to the low-protein diets to meet the nutritional requirements of the rats [11]. Table 1 summarizes the ingredient composition and nutrient levels of the diets. A preliminary experiment was conducted before this study to explore the effect of decreasing dietary protein levels (20%, 15%, 10%, 5%, and 0% casein) on the growth performance, whole-body nitrogen balance, and skeletal muscle protein turnover of adult rats. We found that the growth of rats was extremely inhibited when the dietary casein level decreased below 10% (P < 0.01); the nitrogen deposition (P < 0.01) and protein turnover of skeletal muscle(P < 0.05) also decreased significantly (data were not published). Thus, the casein level of the low-protein diet was 10% in this experiment.
《Table 1》
Table 1 Composition and nutrient levels of experimental diets (as-fed basis).

1 The mineral premix supplied per kilogram of feed: copper, 5 mg; iron, 50 mg; zinc, 50 mg; manganese, 50 mg; selenium, 0.15 mg; iodine, 0.15 mg; calcium, 13 g; phosphorus, 0.36 g; magnesium, 0.5 g; potassium, 3.6 g; and NaCl, 3.6 g.
2 The vitamin premix supplied per kilogram of feed: vitamin A, 4000 IU; vitamin D, 1000 IU; vitamin E, 150 mg; vitamin K, 1 mg; thiamin, 6 mg; pyridoxine, 6 mg; nicotinic acid, 30 mg; folic acid, 2 mg; D-calcium pantothenate, 16 mg; vitamin B 12 , 0.25 mg;
and biotin, 0.2 mg.
3 CON: a 20% casein diet.
4 R: a 10% casein + 0.44% alanine diet.
5 RL: a 10% casein + 0.87% leucine diet.
During the experiment, which lasted for 11 d, the rats were free to access water and their own diets, while we recorded the rats feed intake and body weight. On the 10th day, all rats were fasted overnight for 12 h. On the morning of day 11, rats were re-fed their own diets for 1 h. Thereafter, all rats were injected via the tail vein with a flooding dose of L-phenylalanine (Phe) (150 μmol/100 g body weight (BW)) containing L-[ring- 2H5 ]-Phe (Cambridge Isotope Laboratories, Inc., Andover, MA, USA) at 40% according to the technique of Mao et al. [12]. The injections were finished within 5–10 s. After half an hour, the rats were killed and blood samples were collected from the abdominal aorta of the animal using vacutainer tubes coated with ethylenediaminetetraacetic acid (EDTA) (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA). The blood sample was then centrifuged at 2000g (g = 9.8 m·s –2 ) at 4 °C for 20 min, after which the supernatant was collected and stored at −20 °C for later free amino acid analysis. Soleus and gastrocnemius muscles were harvested and weighed, and then stored at −80 °C until determination of protein synthesis. Mixed skeletal muscles from the hind limbs were also stored at −80 °C for analysis of 4E-BP1 and S6K1 phosphorylation. The abdominal adipose tissue was gently separated and weighed and these values were considered when discussing the animals’ body fat.
《2.2. Chemical analysis》
2.2. Chemical analysis
The content of the experimental diets, such as crude protein, calcium, and total phosphorus, was measured in accordance with Association of Official Agricultural Chemists (AOAC) protocols. Highperformance liquid chromatography (HPLC, Hitachi L-8800 Amino Acid Analyzer, Tokyo, Japan) was used to measure the concentrations of free amino acids. Plasma amino acids were measured by a L-8800 Amino Acid Analyzer (Hitachi, Tokyo, Japan), using detailed protocols described in a previous study [13].
《2.3. Determination of protein synthesis》
2.3. Determination of protein synthesis
The flooding-dose method was used to measure the protein synthesis of skeletal muscles [14]. Samples were prepared and measured as described by Mao et al. [12]. Frozen muscle tissue was then ground to fine powder in liquid nitrogen. Next, the protein was precipitated by cold perchloric acid (4 °C, 30 g·L−1, total volume determined according to 1 mL/50 mg muscle tissue). The supernatant and protein pellet were treated according to the methods in a previous study [15]. The concentration of L-[ring-2H5 ]-Phe was analyzed by gas chromatography-mass spectrometry (GC-MS, Agilent Technologies, Beijing, China).
Fractional synthesis rates (FSRs), which are also known as protein synthesis rates, for skeletal muscle were measured as follows: FSR (%·d −1 ) = (EBound × 1440 × 100%) / (EFT × t). FSR is the percentage of protein renewed in a day. EBound is the isotopic enrichment (%) of the tracer Phe in the protein pool; EFT is the enrichment of the tracer Phe in the free pool at time t; and t is the exact time (min) of protein labeling between the end of the isotope tracer injection and the time the tissue sample was placed in liquid nitrogen.
《2.4. Western blot analysis》
2.4. Western blot analysis
Antibodies against phosphorylated 4E-BP1 (Thr70) and phosphorylated S6K1 (Thr389), along with β-actin, came from Cell Signaling Technology (Danvers, MA, USA). For homogenization, we placed the samples in a buffer containing 50 mmol·L−1 4-(2-hydroxyethyl)1-piperazineethane sulfonic acid (HEPES) (pH = 7.4), 137 mmol·L−1 NaCl, 1% Nonidet P-40, 10% glycerol, 100 mmol·L−1 NaF, 2.5 mmol·L−1 EDTA, 2 mmol·L−1 Na3VO4 , 1 mmol·L−1 MgCl2 , 1 mmol·L−1 CaCl2 , and 2% complete protease inhibitor cocktail (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China). The samples were centrifuged at 10 000g for 10 min at 4 °C, and the supernatant was collected and saved at −80 °C. A Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA) was used to determine protein concentration. The same quantity of each protein sample was electrophoresed on sodium dodecyl sulfate (SDS)-polyacrylamide gel. Polyvinylidene difluoride (PVDF) membrane (Kangchen Bio-tech, Inc., Shanghai, China) was used to electro-transfer proteins. We mixed the membranes with nonfat dry milk at 4 °C overnight, and then incubated them with specific primary and secondary antibodies (horseradish peroxidase, 1:10 000 dilutions in 5% nonfat dry milk). Band densities were detected with western blotting luminance reagents (Santa Cruz Biotechnology, Inc., CA, USA) and scanned with ImageQuant LAS 4000 (GE, Piscataway, NJ, USA). The optical density of the bands for 4EBP1 phosphorylation and S6K1 phosphorylation were normalized using Alpha Imager 2200 (Alpha Innotech, San Leandro, CA, USA) software.
《2.5. Statistical analysis》
2.5. Statistical analysis
A one-way analysis of variance (ANOVA) procedure was used to analyze experimental data, followed by Fisher’s protected least significant difference (PLSD) test. All analyses were performed using SAS (Version 8.1; SAS Institute, Inc., Cary, NC, USA). Differences among treatments were considered significant when P ≤ 0.05.
《3.Results》
3.Results
《3.1. Growth performances and tissue weights》
3.1. Growth performances and tissue weights
The initial BWs of the rats were not significantly different among the three treatments (Table 2). The final BW and weight gain were higher for the CON group than for the R group (P < 0.05). There was no significant difference between the CON and RL groups in terms of final BW, that is, in weight gain. Feed intake was much more (P< 0.05) in the CON group than in the RL and R groups. The RL diet significantly increased the weight of the gastrocnemius muscle (P< 0.05, Table 2) compared with the R diet. No significant difference was found in soleus muscle weight among the three groups. Compared with the R and RL diets, the CON diet decreased the weight of the abdominal adipose (P < 0.05).
《Table 2》
Table 2 Effects of dietary leucine supplementation in a low-protein diet on performance and relative tissue weights in adult rats.

1 SEM: standard error of the mean; the letters a,bindicate that values with different superscripts within a row are significantly different at P < 0.05.
《3.2. Plasma amino acids concentrations》
3.2. Plasma amino acids concentrations
Compared with the CON diet, the R and RL diets increased the concentrations of the EAAs valine, arginine, histidine, lysine, methionine, threonine, and tryptophan (P < 0.05, Table 3), and also significantly increased the concentrations of the nonessential amino acids (NEAAs) serine, glycine, and alanine (P < 0.05). Compared with the R diet, the RL diet significantly increased the concentrations of leucine, histidine, and glycine (P < 0.05), and decreased the concentration of isoleucine (P < 0.05). Compared with the CON diet, the RL diet increased (P < 0.05) the concentration of leucine. There was no significant difference in the concentrations of isoleucine and leucine between the CON and R groups.
《Table 3》
Table 3 Effects of dietary leucine supplementation in a low-protein diet on the free amino acid concentration in the plasma of adult rats (μmol·L−1).

1SEM: standard error of the mean; the letters a,b,cindicate that values with different superscripts within a row are significantly different at P < 0.05.
《3.3. Protein synthesis》
3.3. Protein synthesis
Compared with the R and RL diets, the CON diet increased the protein synthesis rates of gastrocnemius and soleus muscles (P < 0.05, Fig. 1). Compared with the R diet, the RL diet increased the protein synthesis rate of gastrocnemius and soleus muscles in the adult rats (P < 0.05, Fig. 1).
《Fig. 1》
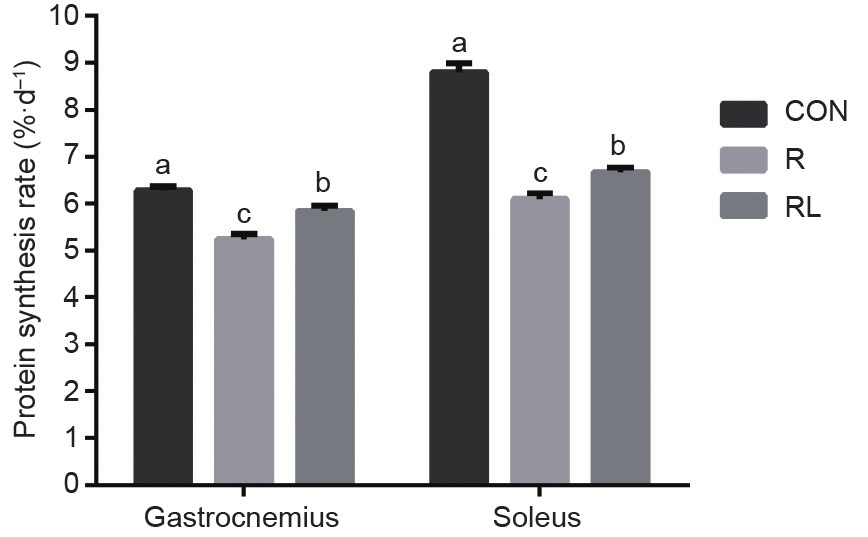
Fig. 1. Effects of dietary leucine supplementation in a low-protein diet on the protein synthesis rate of gastrocnemius and soleus muscle in adult rats. (CON: a 20% casein diet; R: a 10% casein + 0.44% alanine diet; RL: a 10% casein + 0.87% leucine diet; the letters a,b,c indicate that values with different superscripts are significantly different at P < 0.05.)
《3.4. Phosphorylation of 4E-BP1 and S6K1》
3.4. Phosphorylation of 4E-BP1 and S6K1
The phosphorylation of 4E-BP1 was much higher in the RL group than in the R group (P < 0.05, Fig. 2), and much higher in the CON group than in the RL group (P < 0.05). However, the RL group had much higher phosphorylation of S6K1 than the R and CON groups (P< 0.05, Fig. 3), and there was no difference between the CON and R groups.
《Fig. 2》
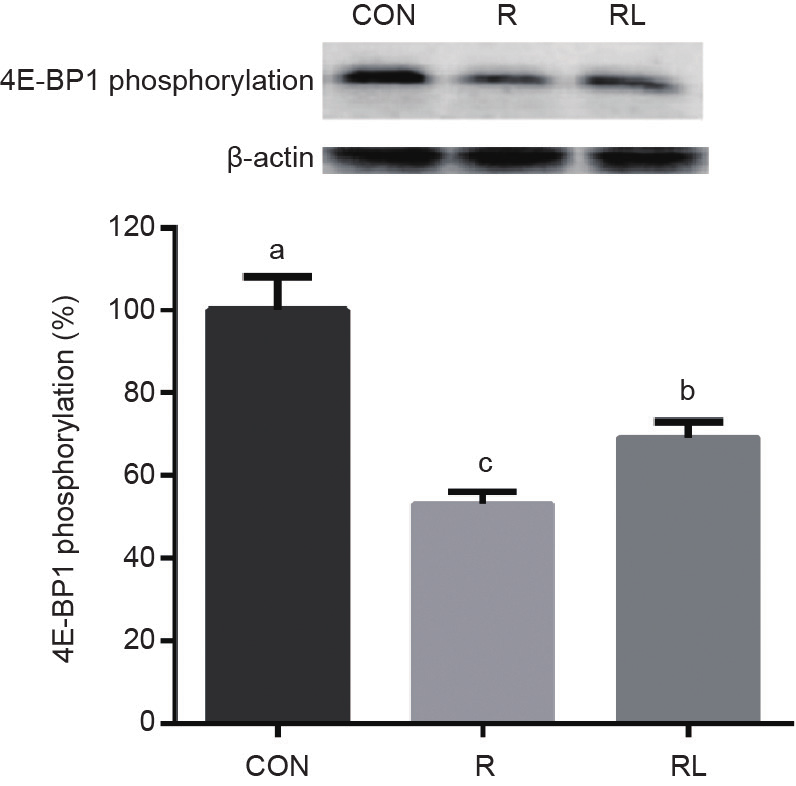
Fig. 2. Effects of dietary leucine supplementation in a low-protein diet on the 4E-BP1 phosphorylation of skeletal muscle in adult rats. (CON: a 20% casein diet; R: a 10% casein + 0.44% alanine diet; RL: a 10% casein + 0.87% leucine diet; the letters a,b,cindicate that values with different superscripts are significantly different at P < 0.05.)
《Fig. 3》
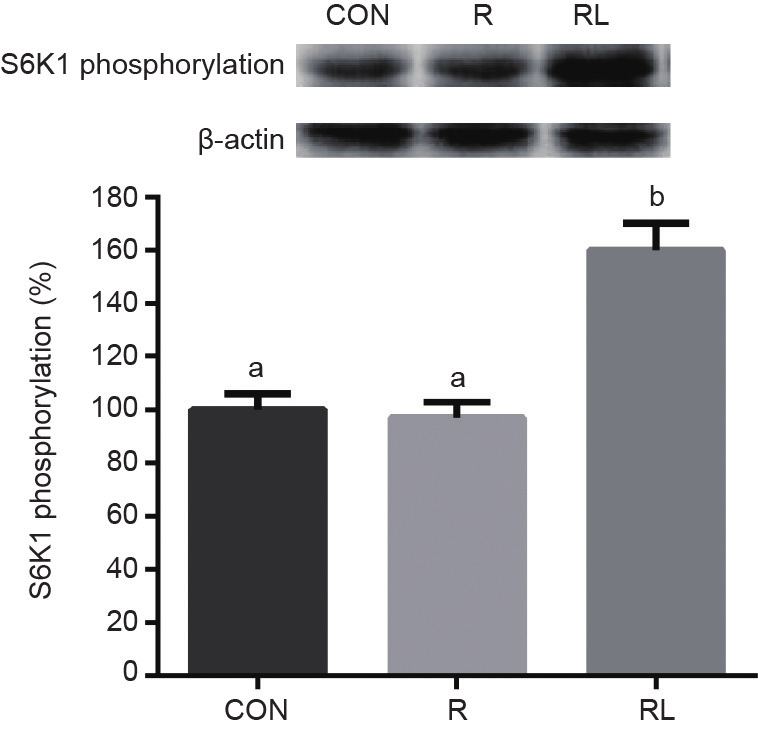
Fig. 3. Effects of dietary leucine supplementation in a low-protein diet on the S6K1 phosphorylation of skeletal muscle in adult rats. (CON: a 20% casein diet; R: a 10% casein + 0.44% alanine diet; RL: a 10% casein + 0.87% leucine diet; the letters a,b,cindicate that values with different superscripts are significantly different at P < 0.05.)
《4.Discussion》
4.Discussion
Protein restriction is typically recommended for patients with kidney and liver diseases, and low-protein diets are widely accepted for farm animals. However, a low-protein diet has been shown to decrease the protein deposition of most tissues in humans [1] and decrease the protein synthesis of neonate piglets [16]. Leucine supplementation in a greatly protein-restricted diet can decrease weight loss and suppress protein degradation in the muscles of rats [5]. However, the effects of leucine on protein synthesis are unclear. Therefore, this study aims to address the effect of chronic dietary leucine supplementation on the protein synthesis of skeletal muscle and the growth performances of adult rats that are restricted to 60% of their protein requirements.
The results showed that feeding rats a protein-restricted diet decreased growth performance such as BW gain. However, given a supplementation of dietary leucine, the RL group was able to attain a BW gain that was similar to the group on the CON diet. Moreover, the RL group had a similar gastrocnemius muscle weight as the CON group, and a much heavier weight than the R group (P < 0.05). It can be argued that the leucine supplement to the protein-restricted diet exerted a beneficial effect on growth performance and muscle mass.
Although the gastrocnemius muscle weight was increased in the RL group, there was no significant difference in soleus muscle mass among the three groups (Table 2), even when there was a significant difference in protein synthesis rate (Fig. 1). This result may be due to the fact that gastrocnemius and soleus are different types of muscle: The gastrocnemius muscles are mainly white muscle fiber (type II fast twitch), whereas the soleus muscles are mainly red muscle fiber (type I slow twitch). The second possible reason is that gastrocnemius muscle in rats has a much higher percent of protein than the soleus muscle: 210 ± 10 mg muscle weight per 100 g BW for the gastrocnemius and 10.4 ± 0.1 mg for the soleus [17]. Thus, even when the protein synthesis rate was higher in the soleus than in the gastrocnemius, the leucine supplementation was likely to affect the muscle weight of the gastrocnemius but not that of the soleus.
The abdominal adipose tissue weight of rats in the RL treatment did not differ from that of rats in the R treatment. However, the abdominal adipose tissue weight of these two groups was greater than that of the CON group. Low-protein diets have been reported to be associated with a reduction in energy loss. Lowering the protein content of the diet reduces the deamination of surplus amino acids and the excretion of urea; it also lowers protein turnover and the heat production of the animal. Therefore, reducing dietary protein concentration increases the energy that is available for tissue deposition. In addition, in order to formulate the low-protein diet, we replaced the casein with corn starch. Starch is more efficient than protein as an energy source in fat deposition. For this reason, feeding animals a low-protein diet causes more lipid deposition than feeding them a high-protein diet. Leucine supplementation did not affect abdominal adipose weight in the present study. It was previously found that leucine supplementation in the diet of young rats increased adipose tissue weight by 25% [18]. Zeanandin et al. [19] found the same result and explained that it may relate to increased food intake or decreased energy expenditure. However, Zhang et al. [20] found that leucine in diets increased energy expenditure and decreased adiposity weight. Therefore, the effect of dietary leucine supplementation on adipose tissue deposition is still unclear and needs further investigation.
The low-protein R and RL diets were supplemented with all the EAAs to meet the nutritional requirements of the rats [11]. The crystalline amino acids present in these diets could enter the animals’ circulation quickly and be utilized by the body after absorption. However, in the CON diet, a minor quantity of crystalline amino acids was added (only L-tryptophan and DL-methionine); in addition, the protein was digested and absorbed, and then converted to free amino acids before it was utilized. For these reasons, the level of postprandial free amino acids in the plasma of most of the rats in the low-protein treatments was higher than in the CON group. Leucine has been reported to have an antagonistic effect on the metabolism of isoleucine [21]. The analyzed amount of leucine in the RL diet was greater than the R diet. Excess leucine probably depressed plasma isoleucine. Several other investigators reported similar results [12,22,23]. Two mechanisms that can explain the leucine-induced isoleucine changes are: ① a transport phenomenon involving the carrier system, which would alter body amino acid distribution; and ② increased branched-chain amino acid (BCAA) oxidation [21].
It has been shown that leucine supplementation in a proteinrestricted diet can promote protein synthesis in the skeletal muscle of piglets in vivo [16]; the same results were observed in humans [24]. In addition, an in vitro incubation media with 0.5 mmol·L−1 leucine was found to stimulate protein synthesis in soleus muscles from starved weanling rats [25]. Our study aligned with these results in that protein synthesis was significantly increased in the RL group as compared with the R group (P < 0.05), although protein synthesis in the RL group was not as high as in the CON group. It can be argued that leucine stimulates protein synthesis with high concentrations of leucine in plasma, which can partly explain why the RL group had a greater protein synthesis rate than the R group (P < 0.05). However, this argument cannot explain why the CON group had a greater protein synthesis rate than the RL group (P < 0.05) even though it had a lower concentration of leucine (P < 0.05). Thus, other explanations are needed. A previous study suggests that following exercise, the effect of chronic leucine supplementation in adult humans may rely on the available substrates, so leucine should be associated with other amino acids [26]. Regarding the composition and nutrients in the experimental diets in our study, the amounts of most EAAs were higher in the CON group than in the R and RL groups (P < 0.05). This explains why the CON group had greater protein synthesis.
Other factors influence protein synthesis for animals with a very low-protein diet, such as feed intake. In the present study, feed intake was much lower in both low-protein groups than in the CON group (P < 0.05). The decreased feed intake may decrease protein intake in both low-protein groups. Kim et al. [27] showed that only sufficient protein intake can maintain higher rates of protein synthesis at whole-body and muscle levels. Another inconsistency between the protein synthesis rate and the muscle weight explanation is that acute protein synthesis is not a suitable indicator when evaluating chronically protein-restricted experiments. One reason is that the magnitude of muscle protein synthesis does not necessarily equate with muscle mass, which also depends on the protein breakdown rate. The second reason is that the evaluation of acute protein synthesis has limitations in short-duration measurements, so longterm measurements of synthesis are advocated in the field [28]. Similar results were observed in piglets [2,9].
Leucine was reported to promote protein synthesis by increasing the phosphorylation of the mTOR complex, S6K1, and 4E-BP1 [29,30]. The mTOR signaling pathway, which is necessary for stimulating translation initiation and enhancing muscle protein synthesis, functions in two distinct protein complexes (mTORC1 and mTORC2) [31]. Activated mTORC1, which has a major role in mRNA translation, promotes 4E-BP1 and S6K1 phosphorylation. Once phosphorylated, eukaryotic initiation factor 4E (eIF4E) is released by 4E-BP1 and then becomes the active eIF4G·eIF4E complex, which binds to mRNA and initiates translation [29]. Phosphorylated S6K1 also takes part in translation initiation [29]. It was recently reported that supplementation with leucine can increase the phosphorylation of both the downstream effectors of the mTOR signaling pathway in normal and intrauterine growth-restricted (IUGR) piglets [32], as well as in neonatal piglets [2]. Similar results were observed in the present study. Another study found that chronically feeding a proteinrestricted diet to weanling pigs decreased muscle protein synthesis by inhibiting 4E-BP1 phosphorylation—but not S6K1 phosphorylation—in mTOR signaling [9]. Similar results were observed in the present study (Fig. 2 and Fig. 3). In addition, the present study indicated that leucine supplementation in protein-restricted diets was not as effective in promoting the phosphorylation of 4E-BP1 as the CON diet, a result that was consistent with protein synthesis results. The results of our study demonstrate that leucine supplementation in low-protein diets enhances the protein synthesis of skeletal muscle through the stimulation of S6K1 and 4E-BP1 phosphorylation.
This discrepancy between our study and earlier investigations may be explained by differences in the animal species tested and in nutritional conditions. Chronic leucine supplementation in the drinking water of young rats increased muscle protein synthesis without any changes in signaling proteins (4E-BP1 and S6K1) [33]. Vary [34] found that oral leucine administration acutely administered with ethanol stimulated myocardial protein synthesis in rats, mainly through increased 4E-BP1 phosphorylation and decreased association of 4E-BP1 with elF4E, while S6K1 activation was not altered. However, Mao et al. [12] reported that leucine can promote the protein synthesis of skeletal muscles in mice by promoting leptin receptor expression. Therefore, further investigation is required to explore other factors in which leucine supplementation in a low-protein diet enhances muscle protein synthesis in adult rats.
《5.Conclusions》
5.Conclusions
Chronic supplementation of leucine in a 40% protein-restricted diet moderately improved the protein synthesis and growth performances of adult rats by means of the mTOR signaling pathway. The leucine also increased the gastrocnemius muscle weight—but not the soleus muscle weight—although significantly different rates of protein synthesis were observed in soleus muscle. This finding may be because gastrocnemius and soleus are different types of muscle, and because acute protein synthesis is not a suitable indicator when analyzing chronically protein-restricted experiments. Further study is needed to determine whether leucine supplementation enhances protein synthesis differently in heart and skeletal muscle when protein is suboptimal.
《Acknowledgements》
Acknowledgements
This study was financially supported by grants from the Beijing Advanced Innovation Center for Food Nutrition and Human Health.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Bo Zhang, Licui Chu, Hong Liu, Chunyuan Xie, Shiyan Qiao, and Xiangfang Zeng declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




