《1. Introduction》
1. Introduction
In 2003, Railsback [1] write an article titled “An Earth Scientist’s Periodic Table of the elements and their ions.” Rather than arranging atoms, Railsback’s table arranges ions into distinct groups, which is more applicable than the conventional periodic table, especially in geology studies. This new table has attracted attention from numerous scholars in different disciplines due to its extensive information and intuitive arrangement. For example, the new table has been used as a benchmark for selecting electrocatalysts [2] and as a guide for ion solubility [3] and has even been used in neuroscience [4], biology [5], genetic science [6], and research on enzyme characteristics [7]. In regards to metallurgy, we have found that the Earth Scientist’s Periodic Table has a close relationship with this form of applied engineering. Many rules that are used for extractive metallurgy, such as chalcophile affinity and anion coordination, are inadvertently summarized in this table. The significant similarities between geochemistry and metallurgy are of great interest, and it is therefore truly worth examining this geochemistry table in order to obtain more information and ideas.
As an applied engineering discipline, metallurgy is used to separate or concentrate elements from ores. Although metallurgy contains a great variety of methods, extraction methods are restricted by the natural environment, which also limit the geochemical behavior of the elements. Therefore, the performance of elements in metallurgical processes is extremely similar to that in geochemical processes. For example, the separation of elements at high temperature in pyrometallurgy is consistent with the fractionation of magmas; the chemical reactions in hydrometallurgy are equivalent to element enrichment in natural water bodies; and oxidation and reduction correspond to atmospheric air and underground conditions, respectively. Railsback’s new table generalizes a regular pattern of geochemistry and, more importantly, unlike the conventional periodic table, summarizes chemistry in terms of charged atoms, considering that most of the earth’s elements exist in the form of anions or cations. The elements in metallurgical processes also have a charged status, which fits exactly with the Earth Scientist’s Periodic Table.
There are dozens of elements on earth and hundreds of kinds of existent substances with different valence states. And the elements are in very uneven distribution. Due to these conditions, a series of geochemical reactions result in a diversity of ore species and mineral resources. Many metallurgical techniques have been learned as a result of geochemistry research. For example, tungsten (W) and molybdenum (Mo) are compatible elements that share similar chemical behaviors, which leads to separation difficulty. Zhao et al. [8–10] proposed a separation technique using the different chalcophile properties between W and Mo, which had previously been revealed through research on the formation of molybdenite (MoS2) and tungsten oxide minerals. As another example, chlorination metallurgy [11,12] is an important method that extracts gold (Au) from ores by the coordination of Au3+ and Cl¯ in [AuCl4]¯ , which is a formation mechanism of Au deposits on earth [13–15]. Similarly, Au, tellurium (Te), bismuth (Bi), and arsenic (As) are highly chalcophile elements [16], and these elements follow the extraction process of copper (Cu) [17].
Conversely, geologists also acquire knowledge from metallurgy. For example, Goldschmidt [18,19] retrieved a great deal of analytical data from the smelting of copper shale ore at Mansfeld, Germany, and used it to develop the classification scheme of elemental affinities. Regardless of whether geochemistry enables new concepts in metallurgy, or metallurgical techniques unexpectedly correspond with geochemical principles, it is clear that metallurgy has many similarities with geochemistry, and that these similarities are expressed in Railsback’s Earth Scientist’s Periodic Table.
With this view in mind, this interdisciplinary paper proposes a geochemical mode of thinking about metallurgy. Using the given properties and existent form of ions from the Earth Scientist’s Periodic Table, this study attempts to identify similarities between metallurgy and geochemistry. The result is an open perspective that attempts to integrate these two disciplines.
《2. Description of the Earth Scientist’s Periodic Table》
2. Description of the Earth Scientist’s Periodic Table
Railsback’s paper [1] provides a detailed description of the Earth Scientist’s Periodic Table, and the perspective used in this study focuses on the table’s metallurgically relevant concepts. The main table consists of six parts, including noble gases, hard cations, intermediate cations, soft cations, element forms, and anions. The typical elements of each parts are listed in Table 1.
《Table 1 》
Table 1 The division of the Earth Scientist’s Periodic Table and the typical elements.

a Noble gases with no ionization.
b “Hard” or “type A” cations with all electrons removed from outer shell.
c Intermediate cations with some electrons remaining in the outer shell.
d “Soft” or “type B” cations with many electrons remaining in the outer shell.
e Elemental forms with zero valence.
f Anions that commonly coordinate with H+ .
The table is crossed by several swaths that are drawn on the basis of ionic potential (z/r ). Some chemical rules are clearly exhibited by this periodic table; for example, it indicates that the coordination ability order for hard cations is F > O > N = Cl > Br > I > S, and the order for soft cations is I > Br > S > Cl = N > O > F. In addition, these patterns not only indicate the absence of CaS and the existence of CaO or CaSO4 in nature, but also display the chemical stability of fluorite (CaF2), which is widely used as a slagging additive in pyrometallurgy. In contrast, the soft cationic minerals are inclined to combine with sulfur (S), and metallurgy’s target for soft cationic minerals is the sulfide form (CuS2, HgS, and PbS).
Eight insets are attached below the main table. Among these, Inset 4 presents the solubility of the oxide minerals of hard cations; Inset 5 exhibits the typical simple oxysalt minerals; Inset 6 shows the melting and decomposition temperatures of the oxides of intermediate and soft cations; and Inset 8 illustrates the solubility of the halides of hard and soft cations. Inset 4 provides a good example of the classification of solubility by indicating that bromellite, corundum, rutile, and baddeleyite, which have extremely low solubility, are the oxide minerals of beryllium (Be), aluminum (Al), titanium (Ti), and zirconium (Zr), respectively, due to the strong attraction of these cations to O2-. This strong combination also leads to separation difficulty in metallurgy, and the desired elements must be extracted by the application of halogens. The combination ability between hard cations and oxygen (O) is discussed below.
《3. The reflection of ionic potential in geochemistry and metallurgy》
3. The reflection of ionic potential in geochemistry and metallurgy
Ionic potential is the ratio of the elemental charge to the radius (z/r ), which is the reference of the swaths drawn across the Earth Scientist’s Periodic Table. The ratios (z/r ) of these lines are 1, 2, 4, 8, 16, and 32, respectively, and the lines cut the table into several parts. For atoms with a positive charge, the higher the ratio is, the stronger that ion’s bonding is with anions. Therefore, the cations in metallic oxides can be broadly divided into three categories: low z/r with weak cation–oxygen bonds (e.g., Na+ , K+ , Ca2+, Ba2+), intermediate z/r with strong cation–oxygen bonds (e.g., Al2O3, TiO2), and high z/r with stronger cation–oxygen bonds (e.g., Cr2O42- , WO42-). This classification is intuitively exhibited in Fig. 1. The ionic potential has a similar effect in both geochemistry and metallurgy due to the ion’s bonding with O2–, which is reflected in the existent form of minerals and in the extractive method used, respectively.
《Fig. 1》
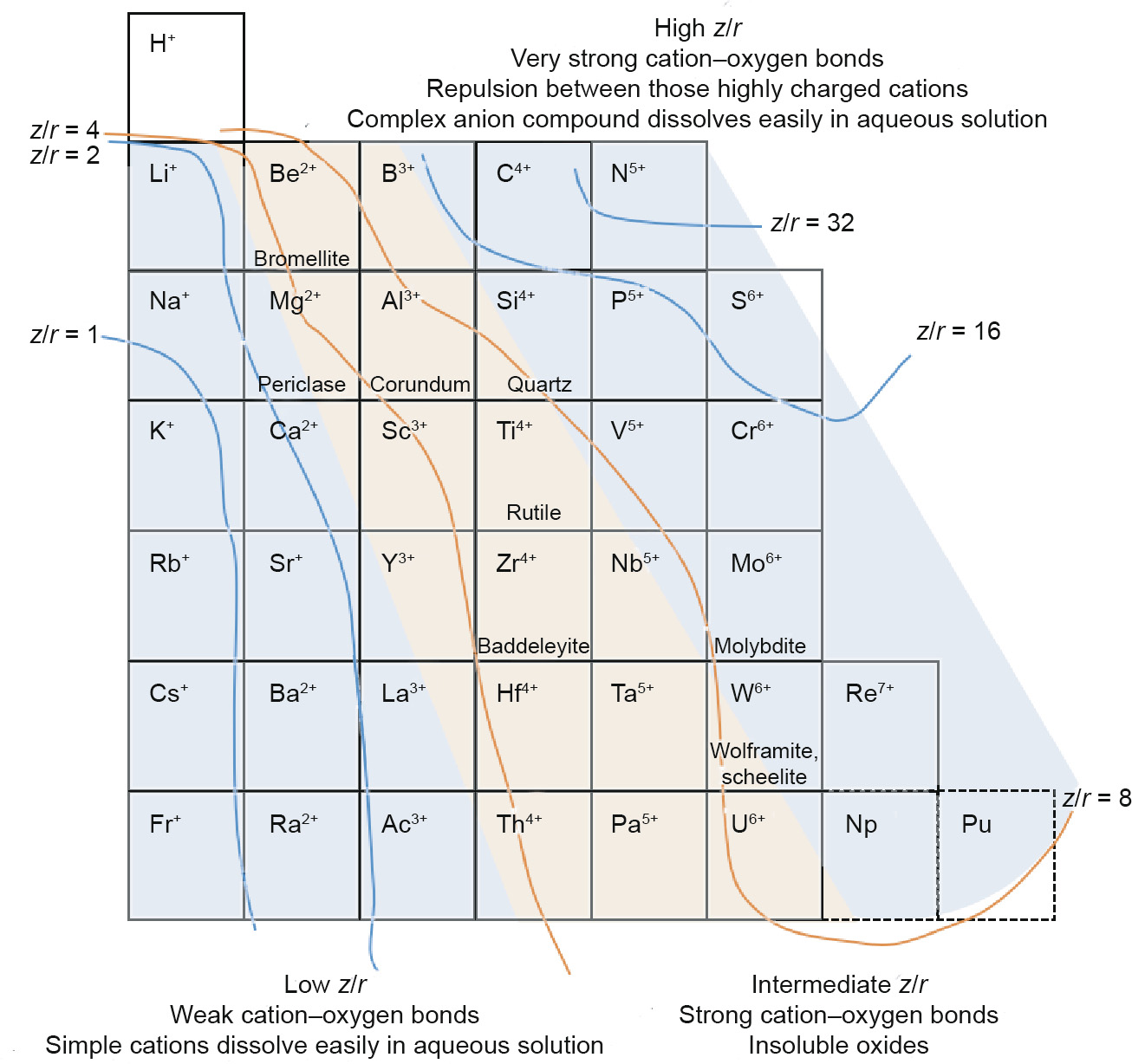
Fig. 1. Distribution of hard cations with graded ionic potential.
On account of this chemical property, in geology, only the intermediate z/r cations exist in metallic oxides (the red swath, e.g., bromellite of Be2+, corundum of Al3+, rutile of Ti4+, and baddeleyite of Zr4+) because of the strong attraction of these cations to O2–, while the low z/r cations are either present in halides or occur in other ores (the left blue swath, e.g., lepidolite of Li+ , feldspar of K+ /Na+ ) due to the weak attraction of those cations to O2–, and the high z/r cations are present in the form of compounds of complex anions (the right blue swath, e.g., chromite of Cr2O42–, wolframite and scheelite of WO42–) due to the very strong bond with O2– and the repulsion between the highly charged cations. The three categories of ionic potential and associated typical minerals are listed in Table 2 [20–29].
《Table 2》
Table 2 Effects of ionic potential for minerals and extracting methods of cations.

The influence of z/r on metallurgy is similar to its influence on geochemistry. The cations that weakly bond with oxygen are easily dissolved in aqueous solution by a hydrometallurgy method, such as leaching Li+ from lepidolite [22] or K+ from glauconitic [30]. The intermediate z/r cations are extracted after the transformation from oxides to halides because it is difficult to separate the cations and oxygen using a suitable method. For example, BeF2 is reduced by magnesium (Mg) at 900–1300 °C [31]; Al2O3, which dissolves in molten cryolite (Na3AlF6), is electrolyzed at 950–970 °C in the Hall–Héroult process [32]; and TiCl4 is successively reduced by sodium (Na) in the Hunter process [33] or by Mg in the Kroll process [34]. The high z/r cations in form of complex anions, such as VO33–, MoO42– and WO42–, due to the very strong attraction between these cations and O2– and the repulsion between the highly charged cations, are leached in aqueous solution.
Metallurgy is not limited to extraction, but also involves concentration, purification, and refining. Metallurgical methods can be divided into two major classes—hydrometallurgy and pyrometallurgy—mainly on the basis of solubility. Fig. 1 exhibits an analogy between geochemistry and metallurgy in terms of hard cations through the trends of ionic potential. Fig. 2 extends these trends to all the elements with cations, including the same elements with different valences [35]. For example, the valence of manganese (Mn) in KMnO4 is +7 and the z/r is between 8 and 16; in comparison, the valence is +2 in MnO and the z/r of Mn2+ is between 2 and 4. The respective states of these two forms of manganese are the complex anion (MnO4–) and the simple cation (Mn2+). Based on the trend from Fig. 1, the water solubility of cations decreases with an increase of z/r from about 1, whereas the water solubility of complex anions decreases with a decrease of z/r from about 32. In other words, compounds with low z/r or high z/r can dissolve in aqueous solution in the state of a simple cation (weak attraction with O2– ) or in the state of a complex anion (very strong attraction with O2– but repulsion between anions). Minerals for which the z/r of the target element lies in the range of 2–8 would be difficult to leach; this applies to minerals such as bromellite, corundum, rutile, and baddeleyite, as mentioned above. In the event of leaching, harsh conditions are required, which would undoubtedly increase the cost. However, there are definitely a few leaching methods aimed at these insoluble minerals that use harsh conditions. For example, bauxite is leached with a high concentration of alkali or high temperature in the Bayer process [36]. The diagrammatic sketch of elements with different charges and z/r is shown in Fig. 2.
In Fig. 2, the reciprocal slope of the dotted line represents the ionic potential, which determines the bond strength with oxygen. Oxides with weak oxygen bonds can easily separate in aqueous solution; separation becomes difficult as the bonds strengthen, and the compounds of the solubility of complex anions decrease as the repulsion weakens. Therefore, based on both theoretical analysis and actual cases, most pyrometallurgy methods are centralized in the intermediate zone of z/r , while hydrometallurgy processes are distributed at both ends of the range of z/r .
《Fig. 2》
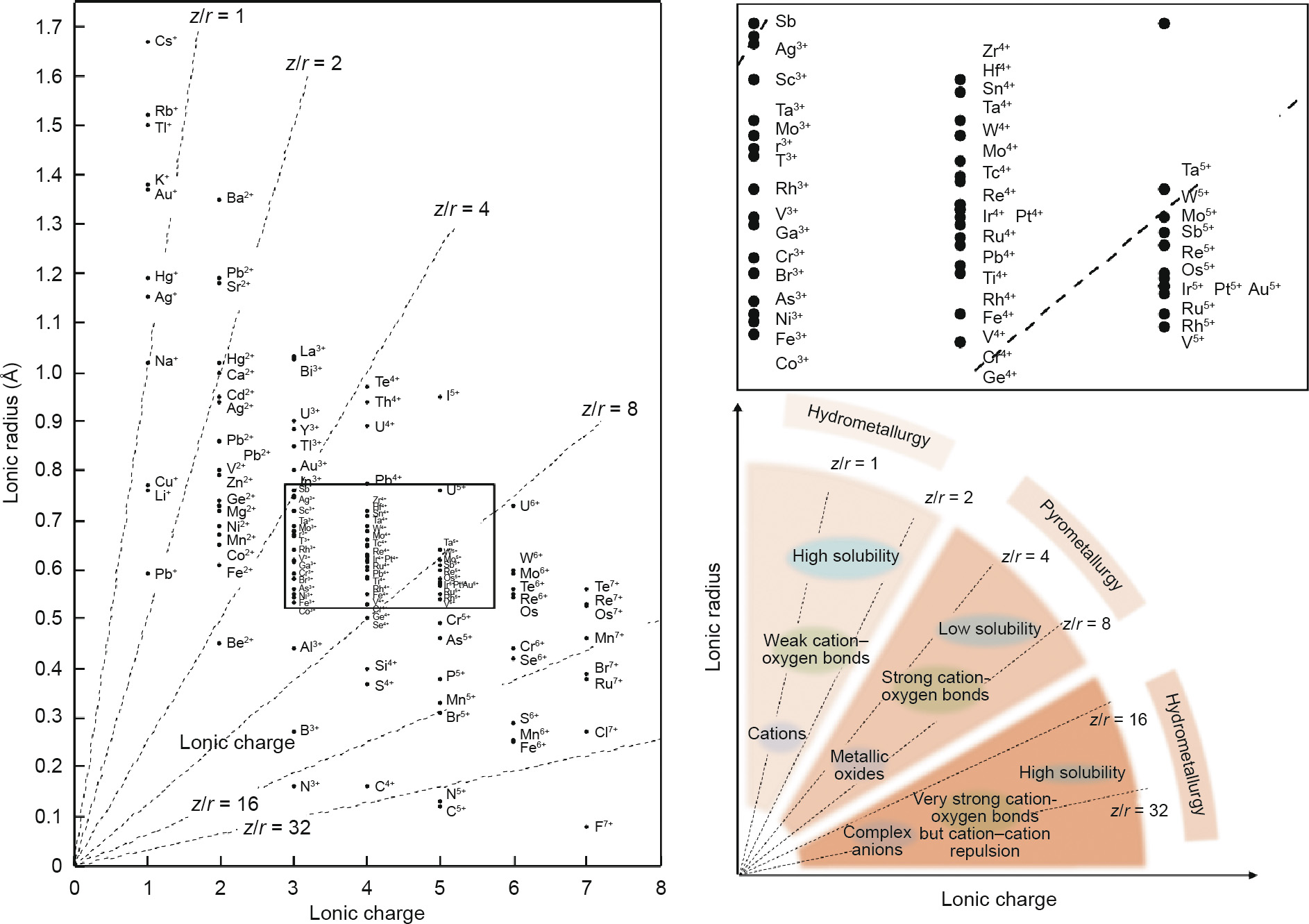
Fig. 2. The ionic potential of elements with different charges and the regularity of the ionic potential map.
As an energy metal of the 21st century, lithium (Li) is extracted chiefly from lepidolite or spodumene by roasting and leaching [37]. In fact, as a low ionic potential element, Li+ has good solubility in water, such as in salt lakes, and the endowment of Li+ in individual brine deposits (1.45 Mt Li per deposit, on average) is much more abundant than that in pegmatite deposits (0.11 Mt Li per deposit, on average). In fact, the Li in brine deposits (21.6 Mt in just four countries, Argentina, Bolivia, Chile, and China) makes up about 70% of all Li resources (31.1 Mt) [38]. Therefore, the trends for Li metallurgy are turning from the pyrometallurgy of Li ores [22] to the hydrometallurgy of Li salt lakes [21,39,40], thereby demonstrating that element geochemistry is leading the development of Li metallurgy.
《4. A metallurgical interpretation of elemental affinity》
4. A metallurgical interpretation of elemental affinity
The classification scheme for elemental affinities was first suggested by Goldschmidt in 1922, after his careful study of the elemental compositions of meteorites and smelting products [41]. He classified the elements into several categories, including siderophile (iron-loving), lithophile (rock-loving), chalcophile (sulfurloving), and atmophile (atmosphere-loving) [19]. These categories were also roughly cited and incorporated in Railsback’s work (Fig. 3) [1]. For example, Cu2+/Cu+ , Ag+ , Hg+ /Hg2+, Tl+ , Pb2+, Bi2+, Sb3+, and As3+ are labeled as “S,” meaning sulfide, in Railsback’s table to categorize them as simple sulfide minerals; coincidentally, they are also placed within the chalcophile zone in Goldschmidt’s scheme. However, many elements occur in more than one valence state and exhibit various affinities. In this regard, Railsback’s table has more advantages of subdivision than Goldschmidt’s scheme. For example, As3+ is strongly chalcophilic, whereas As5+ is a distinct lithophile under oxidizing conditions; Mo4+ is a chalcophile in the form of MoS2 under natural conditions [42], while Mo6+ is a lithophile in MoO42– (molybdate). Therefore, considering the changeability of valence states in chemical reactions, Railsback’s table is more practical for applications.
《Fig. 3》
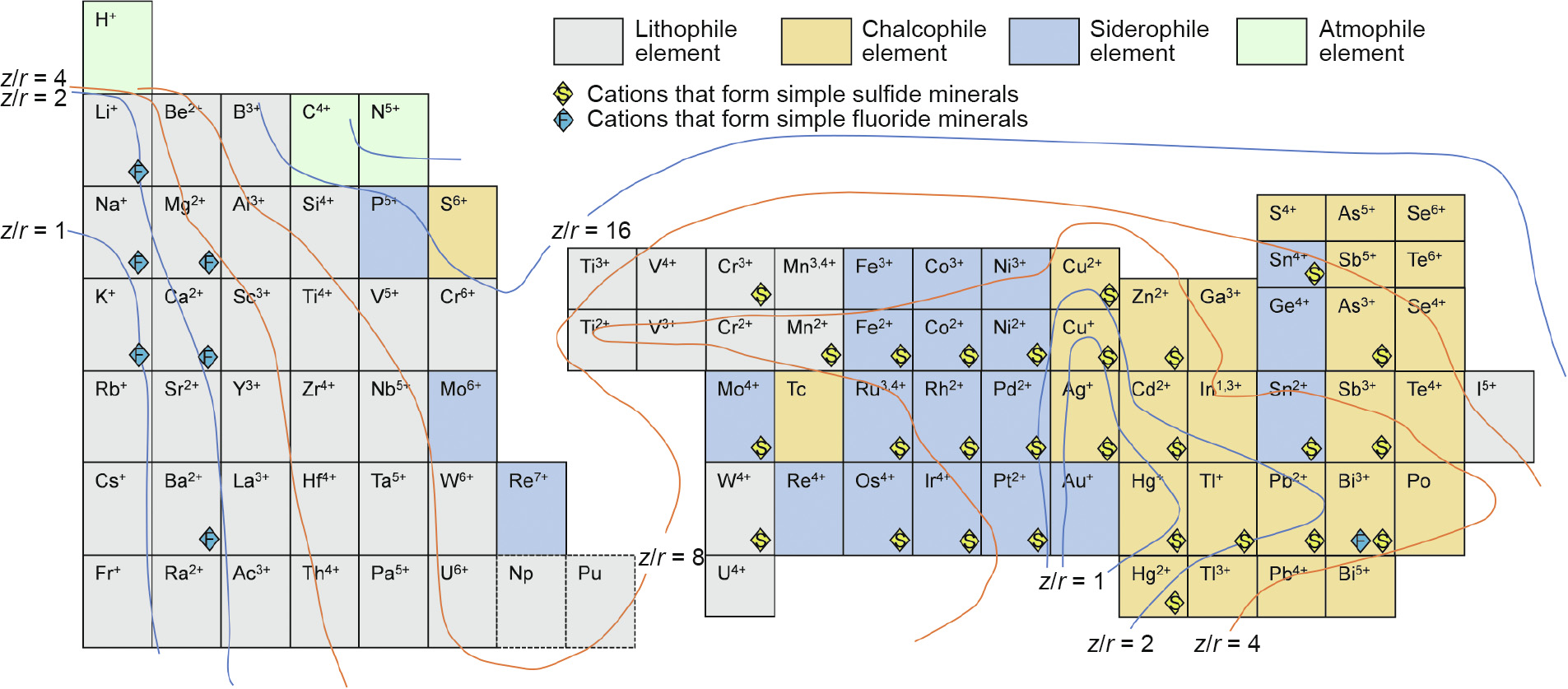
Fig. 3. Geochemical affinity of common elements in the Earth Scientist’s Periodic Table.
Oxidation–reduction reactions are common methods in metallurgical processes. In most cases, the affinity of an element changes with the valence fluctuation, and the elements transfer into another phase, resulting in concentration or extraction. Therefore, studies on elements’ affinities with different charges would be conducive to guiding the migration of elements in metallurgical processes. As the conditions found on earth are both complex and diverse, a series of geochemical cases was summed up for reference. Although Goldschmidt’s scheme does not include the ionic state, that classification is still meaningful, especially for pyrometallurgy. To be specific, the four affinities are classified as follows (Fig. 4) [43–49]: ① Lithophiles are identical to the slags (or “slag”) in metallurgy, which include blast slag, steel slag, and slags from secondary metallurgy; these are mainly comprised of two or more of Al2O3, SiO2, FeO, MgO, CaO, or other metallic oxides. ② Chalcophiles resemble the mattes (Cu, nickel (Ni), or cobalt (Co) mattes), which are generally represented as Cu2S, Ni3S2, CoS. ③ Siderophiles appear to be equivalent tometals or metal alloys. ④ Atmophiles are analogous to the metallurgical gas phase (e.g., H, C, N, O, and inert gases that easily enter into the gas phase in the form of CO, CO2, H2O, SO2, N2, and Ar). By means of geochemistry, elemental affinities are widely used in metallurgy. The three most common examples are given below.
《Fig. 4》
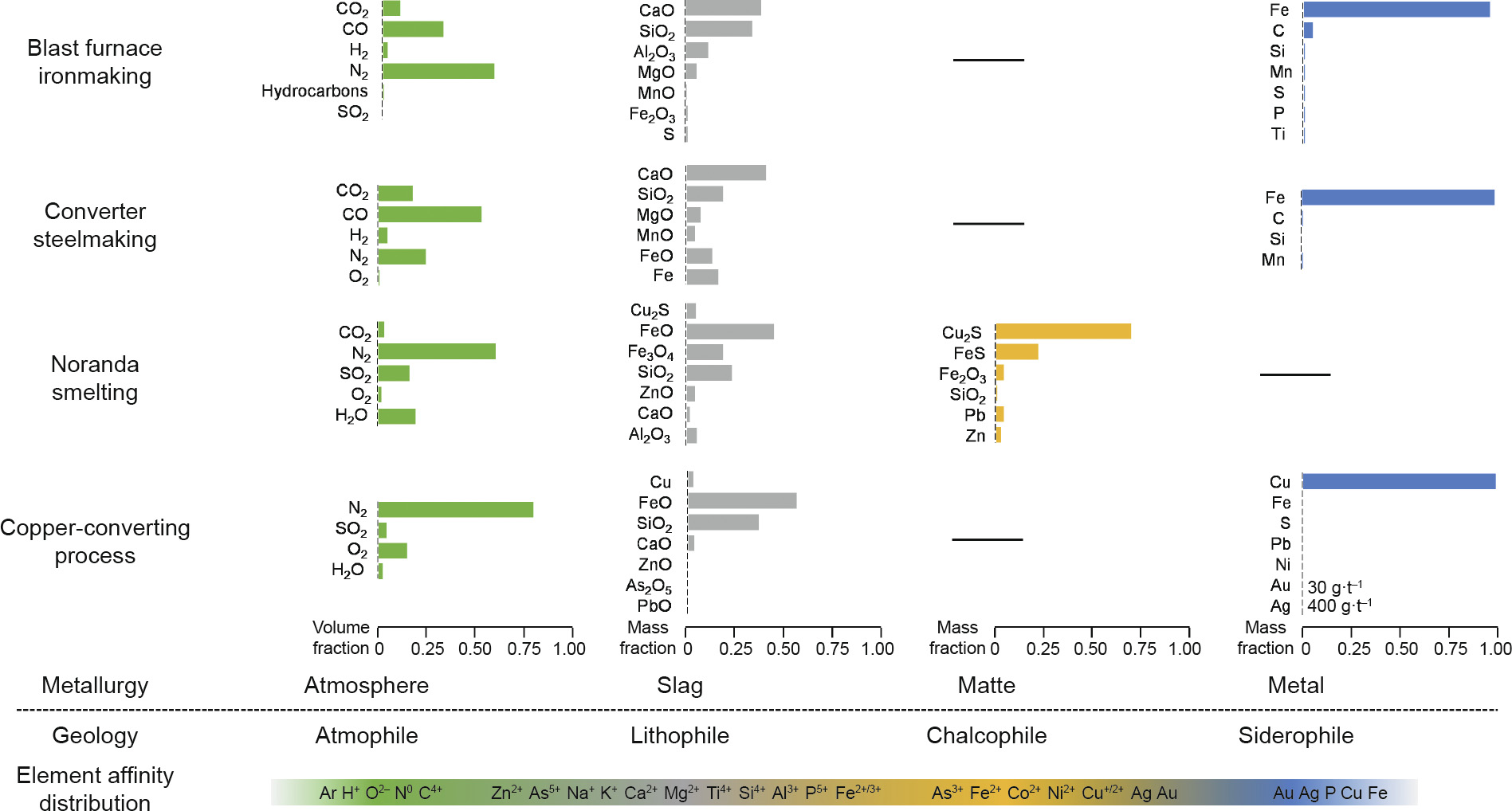
Fig. 4. Element affinity in geology and metallurgy. Blast furnace [43–45]; steelmaking [46–48]; Noranda [49]; copper converting [49].
In Goldschmidt’s scheme, shown in Fig. 3, phosphorus (P) is a siderophile element. The grading basis is difficult to determine, but it is reasonable from the metallurgical view. Due to hightemperature carbon thermal reduction, almost all of the P contained in a blast furnace would be reduced and then dissolve into the iron melt [50]. However, P has a negative effect on the cold, short behavior of steel; thus, one of the steelmaking tasks is dephosphorization [51,52]. In a converter steelmaking process, there are two main phases: the molten slag (Al2O3–SiO2–CaO– FeO) and the molten steel (Fe). Unlike ironmaking in a blast furnace, the P is transferred from the iron melt to the slags by means of oxygen blasting. As the case stands, in a blast furnace, the P behaves a great deal like a siderophile; nevertheless, through oxidation, the siderophile P0 in iron melt changes into the lithophile P5+, which has higher affinity with slags, allowing the removal of P from the molten iron to be achieved. This example, which is shown in Fig. 5, illustrates how, with different valences, one element can have different affinities.
《Fig. 5》
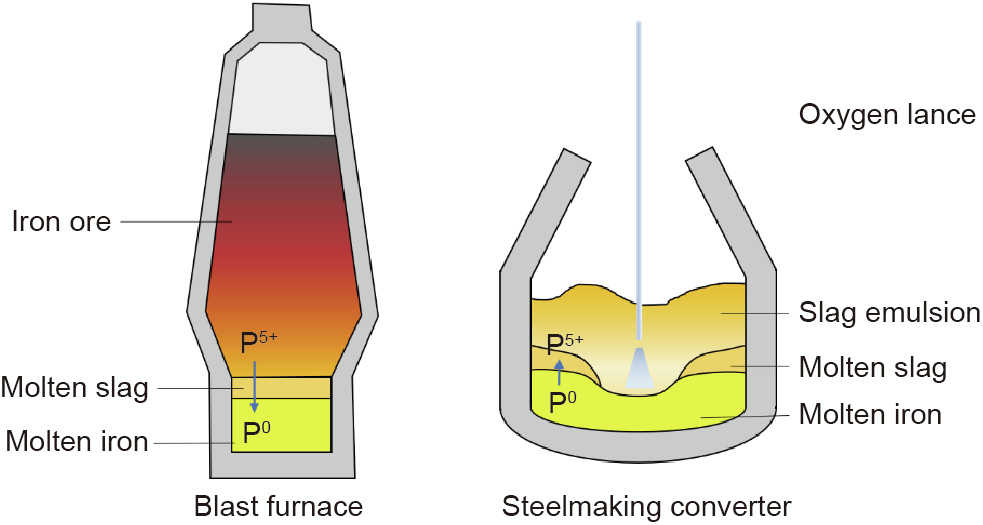
Fig. 5. The transformation and migration of P in ironmaking and steelmaking.
A similar example involves the chalcophile and lithophile states of As. In Goldschmidt’s scheme, As is classified as chalcophile, but in Railsback’s table, only As3+ forms sulfide minerals, and As5+ is closer to being lithophile. This inference is confirmed in matte smelting (similar to Noranda smelting) and in the copperconverting process. As3+ (like As2S3) commonly exists in many Cu ores as an associated element with little industrial value [53], and thus enters into the Cu matte smelting process [54]. As shown in Fig. 6, there are two main phases in matte smelting: the matte (Cu2S–FeS) and the slags (SiO2–Al2O3–CaO–FeO). Most of the As tends to exist in the matte, which is a reflection of its chalcophile nature. However, in the converting process, As is oxidized to As5+ (Eq. (1)), and then migrates from the matte to the slags, or even spreads into the atmosphere in the form of dust [55]. This behavior indicates that As is not only a chalcophile element; it also turns from chalcophile to lithophile with changes in valence. This behavior of As is somewhat embodied in the Earth Scientist’s Periodic Table, because only As3+ is labeled with “cations that form simple sulfide minerals.”

In Goldschmidt’s scheme, Mo is siderophile and W is lithophile. In contrast, only Mo4+ and W4+ are marked as “S” in Railsback’s table, while Mo6+ and W6+ are not. This choice may be inspired by the fact that the major ore of Mo is MoS2 and those of W are scheelite (CaWO4) or wolframite ((Fe,Mn)WO4). However, even though the valence of both W and Mo is +6, Mo is more chalcophile than W. Helz et al. [56] and Mohajerin et al. [57] introduced a mineralization process where, in the anaerobic environment of the deep sea and in the presence of H2S, MoO42– converts to thiomolybdates, which coprecipitate with Fe2+ in the form of Mo–S–Fe (Fig. 7). Based on this difference in element affinity, a method [9] has been proposed to remove Mo from a W aqueous solution. In this removal procedure (Fig. 8), S2– or HS– is added as a vulcanizing agent, which transforms the MoO42– into MoOx S(4–x )2– or even MoS42– ; the thiomolybdates can then be removed by coprecipitation with Fe2+, Cu2+, or Co2+. Both Mo mineralization in natural bodies of water and the metallurgical process of Mo removal can be expressed by Eq. (2).
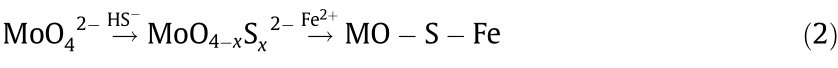
《Fig. 6》
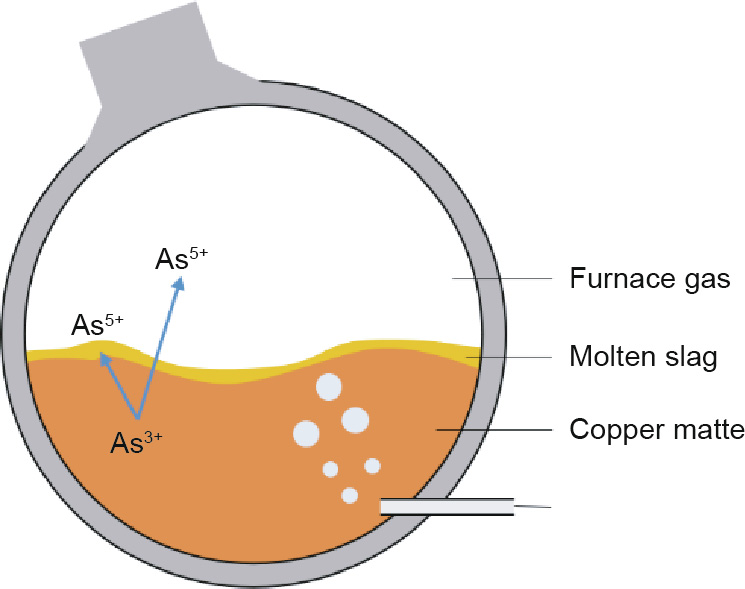
Fig. 6. The transformation and migration of As in the Cu matte converting process.
《Fig. 7》

Fig. 7. Model of Mo scavenging in an anaerobic coastal basin [56].
《Fig. 8》

Fig. 8. Removal of Mo from a W-containing solution through the formation of thiomolybdates.
As shown in Fig. 4, the key compounds of iron (Fe) and Cu in metallurgical processes are generalized to explain element affinities in metallurgy. It can be seen that there are three phases besides the atmospheric phase, and that element migration occurs between the three phases. A regular tendency in pyrometallurgy processes is that the lithophile elements form oxide slags, the chalcophile elements prefer forming matte, and the siderophile elements are inclined to enter into the metal melt (not only iron melt). In addition, the same element with different valence states has different affinities.
In a particular example, there are three phases in the conversion process of Cu–Ni sulfide, as shown in Fig. 9: namely, oxide slags, high Ni–Cu matte, and Ni–Cu metal, where the slag is composed of FeO–SiO2–CaO and the matte consists of NiS and Cu2S. The remarkable thing is that the platinum group elements (PGEs; e.g., Pt0 and Pd0 ) and Au0 , which are siderophile elements, concentrate in the Ni–Cu metal composite [58]. Before the formation of the Ni– Cu alloy, the content of Au, silver (Ag), and PGE are in the oxidizing slag, prior to entering into the matte. With the blowing of oxygen, the primary Ni–Cu alloy phase appears, and the PGE and Au/Ag preferentially enter into the Ni–Cu alloy; this leads to the phenomenon of element migration, in which the noble metals (PGE and Au/Ag) (551.01 g·t-1 ) are a dozen times more concentrated in the alloy than in the matte (28.45–88.7 g·t-1 ) [59]. The Ni–Cu alloy can then be separated for another sulfide melting process; the PGE (Pt, Pd, Ru, Os, Ir, and Rh) in the secondary alloy, which is used for the extraction of precious metals, is concentrated by 5–8, 10–15, 7–14, 3–5, 1–3, or 6–9 times, respectively, in comparison with the primary Ni–Cu alloy [60]. This is a comprehensive exemplification of the application of geochemistry element affinity in pyrometallurgy, which demonstrates that Au/Ag and PGE have a first-priority affinity to the metal phase and a second-priority affinity to the matte phase.
《Fig. 9》
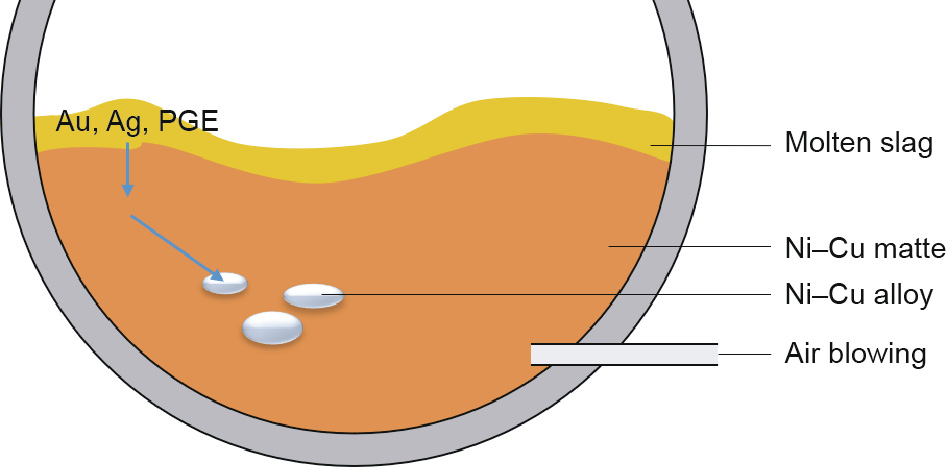
Fig. 9. The concentration behavior of Au, Ag, and PGE in the Ni–Cu matte converting process.
《5. Fluorine and chlorine affinity in hydrometallurg》
5. Fluorine and chlorine affinity in hydrometallurg
Besides O2– and S2–, the halogens (F–, Cl–, Br–, and I– ) are common anions in nature, and F– and Cl– have been shown to play important roles in the migration of elements, especially in hydrothermal mineralization [61,62]. Due to the strong electronegativity of F– and O2–, the fluorine-bearing minerals are distributed in the “hard cations” [63] regions in the Earth Scientist’s Periodic Table, such as CaF2, topaz (Al2SiO4·F2), cryolite (Na3Al2[LiF4]3), and avogadrite ((K,Cs)BF4). In comparison, the chalcophile elements (Cu+/2+, Ag+ , Pb2+) prefer chlorine-bearing minerals, such as botallackite (CuCl2·3Cu(OH)2) and kerargyrite (AgCl). The solubility trends [64] of sulfides and halides can represent the affinities between metallic cations and simple anions to some extent. As shown in Fig. 10, the oxygen-loving cations (Li+ , Na+ , K+ , and Mg2+) show a gradual decline in combining ability in the order of F–, Cl–, Br–, I–, which is embodied in a solubility increase; the sulfur-loving cations (Hg2+, Ag+ , Cu+ , Tl+ , and Pb2+) show a reverse trend toward a solubility decrease (except AgF) [65]. The hard/soft acids and bases (HSABs) principles [66,67] explain a great deal of practical geochemistry phenomena. For example, F– is solidified with hard cations in the earth’s crust, whereas most Cl– and basically all Br– and I– exist in natural aquatic systems [68]. Another example is that chlorine-/sulfur-loving cations easily form complexes with Cl– and S2– (e.g., AuCl4–, [AuS]–, [CuCl2]– ). The bonding between cations and halogens has a great effect on miner alization, and this solubility principle in terms of bonding applies equally to mineral processing. Since hydrometallurgy also mainly involves metallic cations and simple anions in aqueous solutions, the HSABs principles or F–/Cl–-loving classification is also applicable. The following examples may explicate this similarity.
Due to the relatively strong bonding between tantalum (Ta), niobium (Nb), and oxygen, their refractory metallic oxides (Ta2O5, Nb2O5) are difficult to leach by conventional acid or alkali. For this reason, HF is used for dissolving by transforming the oxides into fluorides (H2TaF2, H2NbF2) based on the principle that the oxygen-loving cations also have an affinity with fluorine. Another example is that Cu+ is usually added to the aqueous solution for the removal of Cl–—that is used in an electrolyte purification process [69]—which demonstrates that pro-sulfur elements also have chloride affinity. Furthermore, Au+/3+ not only coordinates with Cl– and S2–, but also dissolves with polysulfides in the form of Au+/3+ complexes, which is consistent with the fact that sulfur-loving elements easily form versatile complexes with Cl–, S2–, NH4– , and CN– .
In addition, as Fig. 10 indicates, metallic cations with chlorine affinity have a greater affinity with Br– and I–, which may indicate that fluorine and chlorine affinities are part of the halogen affinity of hard and soft cations.
《Fig. 10》
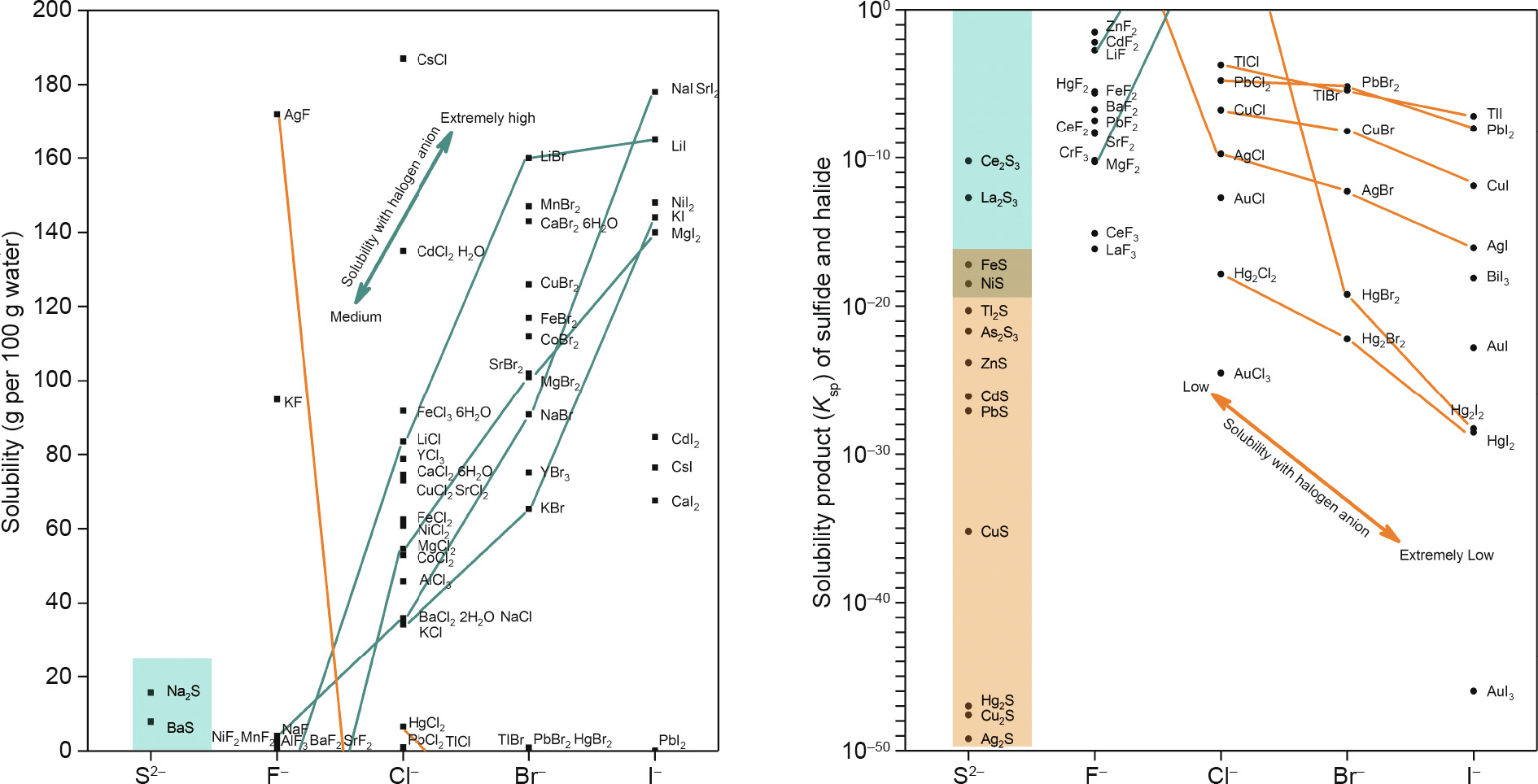
Fig. 10. Solubilities of common sulfides and halides.
As one of the chlorine-/sulfur-loving cations, Au3+ has a strong complexing ability with Cl– . In nature, there is a migration of Au (Fig. 11) in which Au is oxidized by O2 and complexes with Cl– in form of AuCl4– (Eq. (3)). It then flows with liquid until it meets a reducing agent, such as Fe2+, and the Au3+ precipitates as Au0 (Eq. (4)).
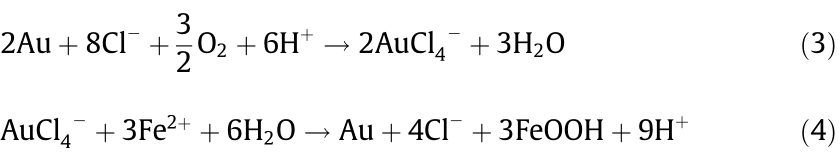
Similarly, the extraction of Au by hydrometallurgy consists of two parts: leaching and precipitating [70,71]. Au-bearing minerals are leached with chloride-containing solutions by blowing Cl2 (Eqs. (5) and (6)) and then precipitating in the metal powder reduction process (Eqs. (7) and (8)). The reactions in these two processes are shown below.
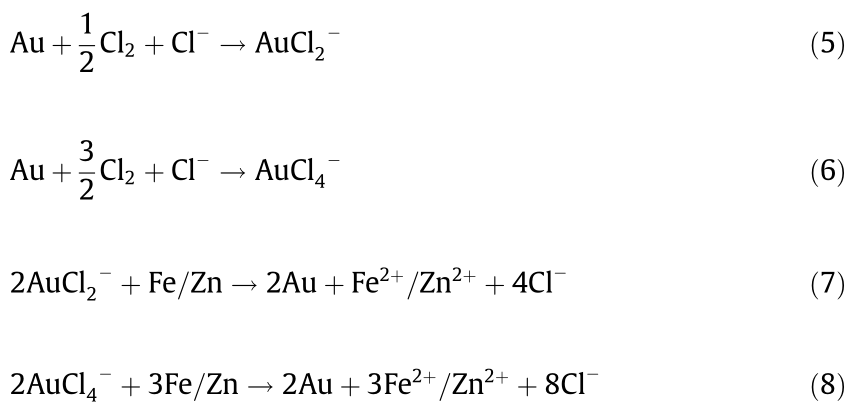
Although the natural processes of geochemistry and the artificial processes of metallurgy belong to two different disciplines, the striking similarities in the examples of Au migration provided above prove that the same chemical mechanisms occur in both.
《Fig. 11》
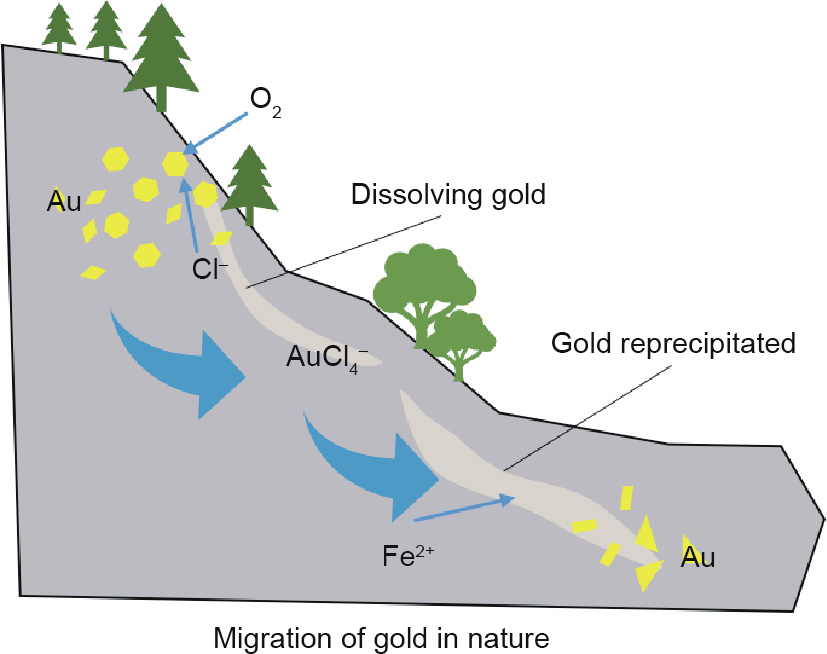
Fig. 11. The dissolving and reprecipitating process of gold with O2, Cl–, and Fe2+.
《6. Summary and conclusion》
6. Summary and conclusion
The Earth Scientist’s Periodic Table is a reinterpreted periodic table that provides a set of laws for metallurgy. The geochemical rules in this new table correlate with metallurgical methods, particularly with regard to charged atoms. The elements’ behavior in natural minerals is similar to their migration and affinity in metallurgy, and has been summarized based on many practical cases as follows:
(1) Cations with high or low ionic potential are most effectively extracted by hydrometallurgy, and cations with intermediate ionic potential are best extracted by pyrometallurgy.
(2) Goldschmidt’s elemental affinity classification is also applicable to elemental migration in metallurgical processes, and that classification would be more accurate if it incorporated different valence states from the Earth Scientist’s Periodic Table.
(3) Fluorine’s and chlorine’s affinities for hard and soft cations, respectively, have an obvious regularity that can be used in extractive metallurgy.
The earth’s natural environment not only yields fundamental resources, but also provides applicable phenomena for metallurgical processes. Utilizing systematic geochemical rules to broaden metallurgical perspectives is a new method of cognition.
《Acknowledgements》
Acknowledgements
This work was financially supported by the Key Program of National Natural Science Foundation of China (51334008).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Fenglong Sun and Zhongwei Zhao declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




