《1. Introduction》
1. Introduction
Breast cancer is the second most commonly diagnosed cancer in women worldwide. In 2018, the global number of new cases exceeded two million, and over 626 000 patients died from the disease, according to GLOBOCAN 2018↑ . (↑ https://www.uicc.org/news/global-cancer-data-globocan-2018) The leading cause of death is metastasis of the breast tumor to distant organs and the resulting dysfunction of the organs. To metastasize, breast tumor cells must invade the surrounding tissue and migrate away from the primary tumor tissue. This motile capacity of cells is driven by forces that cells exert on the extracellular environment and by the mechanical properties of the extracellular microenvironment. The forces of cells can deform and reorganize their microenvironment, and the stiffness of the environment can regulate these cellular forces [1]. The spatial location of cells in the tumor also influences their migratory behavior [2]. Understanding the feedback mechanism(s) through which cellular forces regulate the local extracellular environment, and how the stiffness of the environment regulates cells, will lead to a greater understanding of the development, progression, and metastasis of breast cancer.
The extracellular stiffness, architecture, and organization of tissues have profound influences on tissue function in both healthy and pathological contexts [3]. For example, these properties change during the initiation and progression of cancers, as well as during cell invasion and metastasis to distant sites [4]. Weaver and colleagues, as reviewed by Kumar and Weaver [4], pioneered the field of breast cancer research by highlighting the mechanical causes of the disease. Indeed, the stiffening of breast tissue and the progressive loss of tensional homeostasis can be used to detect tumors [5]. In addition, approaches based on the physical properties of cancer cells can contribute to new treatment strategies and to the development of new diagnostic tools and cancer treatments [6].
Several lines of research have focused on understanding the role of extracellular mechanics in breast cancer invasion, dissemination, and response to treatment, often using approaches based on bioengineered three-dimensional (3D) materials. In this brief review, we describe the mechanical characteristics of breast cancer tissues and recent advances in 3D biomaterials that can mimic the responses of cells to the stiffness, density, and viscosity of the microenvironment. We further highlight the importance of taking the heterogeneity within and between tumors and patients into account in order to model the interactions between tumor cells and the microenvironment, and to guide the development of novel mechano-based therapies against cancers.
《2. Stiffness as a biomarker for breast cancer》
2. Stiffness as a biomarker for breast cancer
Malignant breast tissue is stiffer than normal tissue [7]. This substantial increase in the stiffness of the breast tissue has long been used for palpation-based diagnosis. Breast cancer is a very heterogeneous and complex disease with a wide range of morphological features, immunohistochemical profiles, and unique histopathological subtypes, all of which have specific clinical courses and outcomes [8]. These diverse subtypes can therefore have different and wide-ranging mechanical properties. Invasive ductal carcinoma is the most common form of invasive breast cancer, accounting for more than 50% of breast cancers [8,9]; invasive carcinoma and carcinoma in situ are classified as ductal or lobular, based on the site from which these tumors originate [8].
To provide an overview of the literature showing how the stiffness of breast cancer tissues differs from that of the tissues of the normal breast and non-cancerous lesions, we performed a systematic literature search. Table 1 [7,10–15] provides representative examples from the identified literature showing the ranges of stiffness for different histological types of breast cancer, normal breast tissue, and non-malignant breast lesions. Methods used to analyze tissue stiffness include atomic force microscopy (AFM) and shear wave elastography (SWE), which can be used to differentiate between invasive and noninvasive tumor tissue. Although the stiffness range varies according to the method used, it is clear that breast cancer tissues show increased stiffness when compared with various types of normal tissue and non-cancerous lesions, both in vivo and ex vivo. In addition, the data indicate increased stiffness in invasive cancer compared with noninvasive cancer (Table 1).
《Table 1 》
Table 1 Studies determining the main stiffness characteristics of normal breast tissue, benign lesions, and tumor tissue in the adult human breast, as analyzed by atomic force microscopy (AFM), shear wave elastography (SWE), and B-mode ultrasound (B-US).


The stiffness of body tissues is mainly governed by the stiffness of the extracellular stroma, which is a fibrillar matrix of collagen and other extracellular matrix proteins and molecules. The production and/or crosslinking of these components increases the stiffness and density of the tissue [16,17], which correlates with progression in breast cancer [16,18]. Provenzano et al. [19] showed that the increased density of collagen promotes the initiation and progression of mammary tumors in vivo. They also reported a strong correlation between collagen fibers that were oriented radially from—instead of aligned with—the tumor boundary, and local invasion of the tumor [19]. This increased density and alignment of collagen fibers has been further linked to poor prognosis and can be used as a prognostic marker for these patients [20]. However, whether the density of collagen contributes to prognosis remains unclear. Indeed, a large study of 9232 women diagnosed with primary invasive breast carcinoma from 1996 to 2005 failed to identify any correlation between mammographic breast density and risk of death from breast cancer [21].
In the studies described above, it was not possible to determine whether it is the amount and density of collagen or the tissue stiffness per se that regulates tumor invasion. To determine the relationship between tissue stiffness and tumor progression, Fenner et al. [22] resected tumors from mouse models of breast cancer and analyzed the bulk moduli of the freshly excised intact tumors ex vivo. Unlike previous studies, they reported a clear inverse correlation between the bulk modulus of the resected tumors and subsequent local recurrence and metastasis; furthermore, they reported that tumor stiffness correlated with the amount of collagen in the tissue. It is also important to note that Levental et al. [18] used a mouse model of breast cancer to show that increased collagen crosslinking was related to increased invasion of tumor cells, with no change in collagen levels. Taken together, these findings emphasize that the effects of the extracellular matrix on cell behavior are complex; they also highlight that collagen density, amount, alignment, crosslinking, and spatial organization in relation to the surface of the epithelial component of the tumor should be considered to determine whether compliance can be used as a biomarker for breast cancer.
《3. Extracellular matrix stiffness and breast cancer》
3. Extracellular matrix stiffness and breast cancer
To understand how the mechanical properties of the extracellular matrix regulate oncogenic cell transformation and tumor cell invasion, these properties need to be examined without changing the chemical composition or architectural properties. To this end, synthetic fibrous materials, polyacrylamide hydrogels, and polydimethylsiloxane elastomer-coated substrates have been developed. Chaudhuri et al. [23] showed that normal, MCF10A mammary epithelial cells that are exposed to increased matrix stiffness have a phenotype similar to that of oncogenically transformed cells. This finding is in line with the observation that the stiffness of the matrix in a 3D culture model of breast cancer affects the accessibility of the genome and induces malignancy [24].
Endothelial cell sprouting and blood vessel formation is required for the growth and metastasis of breast cancers. In contrast to epithelial cells, endothelial cells showed decreased proliferation, invasion, and sprouting when exposed to a gelatinmethacrylate and collagen matrix with increased stiffness and constant collagen density [25]. In the same work, Berger et al. [25] reported that increased stiffness resulted in a gradual decrease in cell sprouting at 1.5 and 3.0 mg·mL–1 of collagen I. In the absence of collagen, stiffness promoted cell sprouting, with a peak at 7 kPa; however, cell sprouting was reduced above this stiffness, reaching near zero at 12 kPa.
Collagen type I can influence cell responses to tissue stiffness, and these findings by Chaudhuri et al. [23] and Berger et al. [25] highlight the importance of taking the chemical composition of the material into account at any given stiffness.
In line with these observations, it should be noted that the responses of cells to mechanical cues depend on the type of cell under study [10,26]. The stiffness of extracellular fibers regulates the migration speed of a large variety of cells, such as metastatic MDA-MB-231 breast cancer cells, UM-SCC-74B squamous cell carcinoma cells, HT1080 fibrosarcoma cells, and NIH3T3 fibroblasts. However, the optimal fiber stiffness for the speed of cell migration varies considerably between different cell types [27]. Computational modeling based on experimental data has suggested that cells exhibit their maximal speed of migration at an intermediate range of fiber stiffness, and that the optimal stiffness for maximal speed varies between cell types [26]. This concept is supported by Wang et al. [27], who demonstrated an optimal stiffness of synthetic 3D fibers for the maximum speed of cell migration. It is worth noting that the type of migration observed on these fibers was a ‘‘slingshot” movement rather than the traditional mesenchymal migration. In addition, recent observations highlight the possibility that it is not the increased stiffness of the extracellular environment or of fibers in three dimensions, but rather the increased fiber density that promotes the changes of stromal cells that often are observed in cancer [28,29]. Taken together, these observations emphasize the importance determining the optimal stiffness for a given matrix composition and dimensionality for a specific cell type. Identification of the optimal stiffness for cell migration in vivo and the underlying molecular mechanisms in its regulation would make it possible to identify relevant biomarkers for invasive tumor regions, which could guide the development of novel mechano-based anti-cancer therapies.
《4. The impact of the viscous properties of breast cancer》
4. The impact of the viscous properties of breast cancer
As described above, recent research in bioengineering has led to the development of novel biomaterials that can be used to mimic the elastic modulus and architecture of the tumor microenvironment. However, the tissues in our bodies do not behave like an elastic solid; instead, they have both viscous and elastic characteristics. It is therefore important to consider other mechanical properties, such as viscosity.
Breast cancer tissues have been found to be more viscous, or fluid-like, than tissues from benign lesions [30], which aligns with the observation that the production of hyaluronan, a molecule that governs the water content of tissues, is upregulated in and linked to poor prognosis of breast cancer [31]. Magnetic resonance elastography has demonstrated significant differences in viscoelasticity between malignant and benign breast tumors [30], as well as between glioblastoma and healthy brain parenchyma [32]. MCF-7 breast cancer cells show reduced viscosity and elasticity, which indicates that breast cancer cells are more fluid and ‘‘softer” than their benign counterparts [33]. A further study using MCF-10A, MCF-7, and MDA-MB-231 cells showed that normal breast epithelial cells have greater viscosity than tumor cells; the actin distribution and the greater nucleus-to-cytoplasm ratio of the tumor cells are the two main factors in the determination of cell viscosity [34]. Tumor cell metastasis depends on various factors, which include remodeling of the extracellular matrix and the potential for deformation of the nucleus [35,36]. However, it should be noted that on very rigid substrates, viscosity has little effect on cell attachment and spreading [37]. Viscosity playing a role in the spread of cancer is supported by the observation that while cancer cells are unable to squeeze and migrate through a very rigid pore size environment [35,38], they can migrate through nanoporous matrices that exhibit sufficient mechanical plasticity [39].
Recently developed hydrogels offer the possibility of tuning the stress relaxation or loss modulus independently from the elastic modulus [40–44]. Several cell types respond to viscoelastic substrates as though they were softer than purely elastic substrates of the same elastic modulus [44]. For example, fibroblasts and cancer cells were unable to spread on soft elastic gels; however, they were able to spread on soft viscoelastic gels through the β1 integrin receptor protein, myosin, and Rho, exhibiting robust focal adhesions and stress fibers and enhanced activation of the transcriptional regulator protein YAP, similar to their behavior on stiff and elastic substrates [45]. Increased stress relaxation promotes cell spreading, proliferation, and the osteogenic differentiation of mesenchymal stem cells in 3D culture [42]. These observations align with the observation that viscosity can have a profound effect on cell morphology, adhesion, proliferation, and differentiation [43]. Taken together, these observations indicate that it is important to include both the viscous and elastic properties when developing an understanding of the regulation of tumor invasion and metastasis.
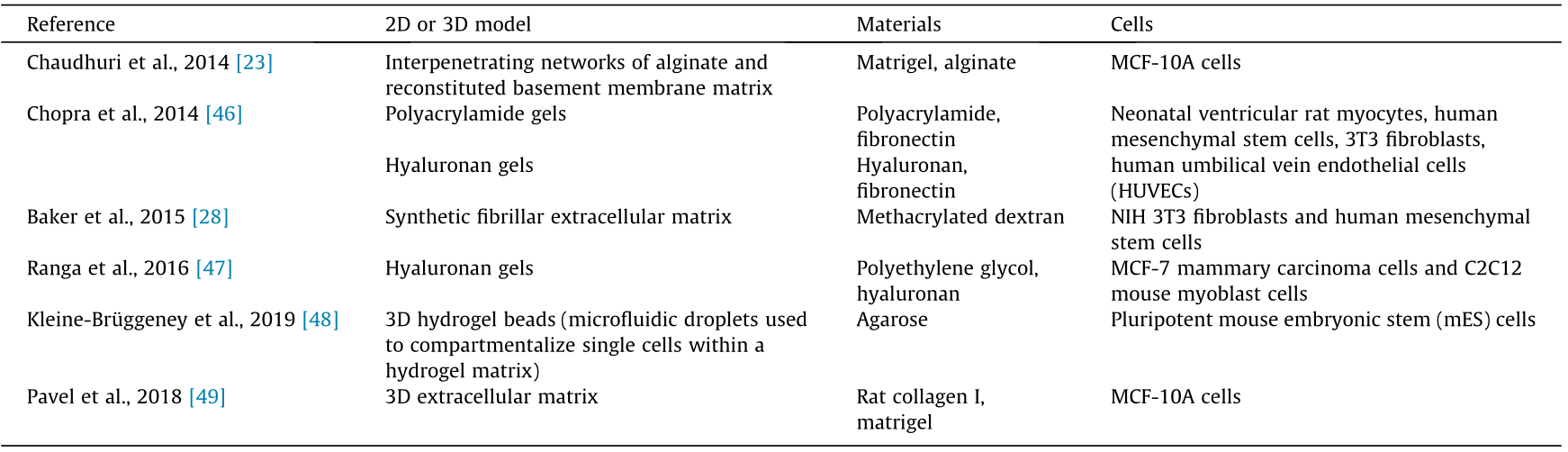
Engineered biomaterial in vitro models with independently tunable viscoelastic properties provide new avenues toward an understanding of the time-dependent aspects of extracellular matrix mechanics in the regulation of cell behavior and metastasis. In addition, these models provide an efficient reductionist approach for studying the impact of different extracellular mechanical cues and a wide range of extracellular proteins on tumor development and progression. For example, chemically defined hydrogels and synthetic fibers can be functionalized with collagen or fibronectin [44], or with arginine–glycine–aspartic acid (RGD) peptides [28,45], to study the impact of elasticity and viscosity on cell adhesion and motility. Table 2 [23,28,46–49] lists examples of preclinical biomaterial in vitro models that can be used to study the impact of extracellular mechanical cues on breast cancer.
《Table 2》
Table 2 Examples of preclinical two-dimensional (2D) and 3D in vitro biomaterial cancer models, including the model, material, and cell type.
《5. The impact of intra-tumoral mechanical heterogeneity》
5. The impact of intra-tumoral mechanical heterogeneity
It is important to note that the same biomaterial model will result in different effects on different cell types [27]; furthermore, the same cell type derived from different patients will respond differently to the same stiffness [50,51]. Therefore, understanding inter- and intra-tumoral heterogeneity at the cellular level can be key in understanding cancer development, progression, and treatment failure.
Most of the aforementioned 3D models have used immortalized cell lines, many of which have been cultured on glass or plastic for decades. These cell lines are therefore likely to have developed properties that facilitate their growth under these nonphysiological—and very stiff—culture conditions. Thus, these cells should not be considered as true representatives of tumor cells in patients, and it is likely that their responses to mechanical cues will differ from those of tumor cells in vivo. It has been observed that glioblastoma cells derived from different regions within a tumor show different mechanical behaviors in standardized controlled settings, which further adds to the complexity of the mechanical heterogeneity of tumor cells and how this heterogeneity can regulate tumor progression and infiltration [50].
Several observations have suggested that the material properties of breast tumors depend on the region of location within the tumor. For example, tumors have a core that is almost as compliant as normal tissue, whereas the invasive tissue at the rim is stiffer [7,52]. The causes of this intra-tumoral heterogeneity remain unclear. It is therefore important to sample and test cells from different tumor regions under standardized conditions. Doing so will assist researchers in understanding how much of the phenotypic variability between cells in different parts of a tumor is due to the cell types themselves, and how much is due to the environment [50].
Although the tumor stroma is the main determinant of the stiffness of tumor tissue, it should be noted that the tumor cells, immune cells, cancer-associated fibroblasts, endothelial cells, and necrotic areas also contribute to the mechanical properties of the tissue. It has been observed that most tumor cells with low migration and invasion potential show a five-fold greater stiffness than migratory and invasive tumor cells [53], which suggests that reduced cell stiffness promotes metastasis. In a ground-breaking finding, Kenny and Bissell [54] reported that a normal extracellular environment can revert the phenotype of an oncogenically transformed cell back to normal, which suggests that the phenotype of the extracellular environment can override the malignant genotype of a cell. It is therefore important to further clarify how much of the behavior of cells is due to gene expression, and how much is regulated by the surrounding environment.
Taken together, the studies described above highlight the need to analyze cells in different stages of oncogenic transformation and from different tumor regions under standardized extracellular conditions, using, for example, fibers with defined mechanical, chemical, and spatial properties. In addition to this intra-tumoral heterogeneity, it should be considered that breast cancer is a heterogeneous disease, and that different breast cancer types have different and unique features.
《6. Identification of biomarkers for molecular diagnosis, patient prognosis, and targeted therapies》
6. Identification of biomarkers for molecular diagnosis, patient prognosis, and targeted therapies
Materials that can mimic the mechanical properties of the extracellular environment would permit the identification of the mechanical conditions required for tumor cell invasion and the underlying molecular mechanisms of this regulation. These mechanisms could then be used to develop novel biomarkers and molecular targets that could be used to control tumor invasion via regulation of the cell mechanics. Berger et al. [55] used a matrix composed of methacrylate gelatin and collagen I to show that cell invasion into stiff matrices depends upon the extracellular protein fibronectin. They further showed that, compared with normal tissue, a domain of fibronectin that is overexpressed in invasive breast cancer cells can promote cell invasion ex vivo [55]. Targeting this domain of fibronectin therefore represents a therapeutic strategy. This finding can also be used to develop diagnostic strategies to identify patients who have more invasive tumors and therefore require more aggressive treatments.
As mentioned above, the phenotype of oncogenically transformed malignant cells can be reverted to a more normal phenotype through normalization of their microenvironment [54]. Therefore, newly developed models can ideally be used to identify the stiffness and viscous conditions [23] and the physiological extracellular ligands that can revert the malignant phenotype of a cell to a more normal phenotype. Although these models offer an excellent opportunity to decouple the mechanical properties from the chemical characteristics of the extracellular matrix, they do not decisively describe whether and how stiffness regulates the progression of breast cancer. We speculate that the conflicting data described herein are due to differences between the cell types used and the chemical compositions of the materials used for the model systems. As described earlier, a problem with the current models of breast cancer is that they mainly depend on the use of immortalized tumor cell lines that do not necessarily represent the tumor cells derived from patients, where tumor cells in vivo are likely to show different mechanical responses. In addition to the standard classification of breast cancer cells from a patient using molecular and clinical markers, classification based on the mechanical properties of breast cancer cells can be used to provide a more personalized and specific diagnosis. Further studies will also allow researchers to understand why treatments aimed at blocking integrin receptors do not effectively block cell invasion, as well as providing novel molecular targets for treatments. The current challenges in the field of bioengineering are to create environments that mimic breast cancer tissue such that the stiffness, viscosity, and architectural cues of the cells can be tuned independently of each other, and that can be used with a wide range of cells from patients.
《Acknowledgments》
Acknowledgments
The authors would like to thank the Weston Park Cancer Centre (University of Sheffield, UK) the Fundação para a Ciência e a Tecnologia (FCT), the Portuguese Government (PEst-OE/QUI/ UI0674/2013) and the Agência Regional para o Desenvolvimento da Investigaçaõ Tecnologia e Inovação (ARDITI), M1420-01-0145- FEDER-000005 Centro de Química da Madeira (CQM) (Madeira 14–20).
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Rasha Rezk, Raquel Marín-García, and Annica K.B. Gad declare that they have no conflict of interest or financial conflicts to disclose.














 京公网安备 11010502051620号
京公网安备 11010502051620号




