《1. Introduction》
1. Introduction
The treatment strategy for rheumatoid arthritis (RA) is to initiate therapy soon after diagnosis, guided by an assessment of disease activity and with the aim of clinical remission [1–3]. Regardless of the advances that have been made in biological disease-modifying therapies, treatment sometimes fails to mitigate disease progression in patients [4], and only about 10% of patients achieve clinical remission in China [5]. Moreover, there is a lack of reliable biomarkers reflecting disease severity and treatment responses, so treatment decisions are mainly driven by clinical symptoms, treatment-specific side effects, patients’ preferences, and costs regarding drug choice [6]. The current clinically used serum biomarkers—namely, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels—provide information about disease activity but are dependent on age and sex [7]. The identification of new RA treatment and biomarkers that can be applied to treatment stratification is therefore an unmet medical need.
Sinomenine (SIN), which is extracted from Caulis Sinomenii, has been approved by the National Medical Products Administration of China and is listed in the National Health Insurance Directory of China for the treatment of RA, although more evidence is still required on its effectiveness and safety. In our previous study, a combination therapy using methotrexate (MTX) and SIN revealed a therapeutic efficacy comparable to that of MTX and leflunomide (LEF), but with fewer side effects [8]. Furthermore, an MTX and SIN combination treatment was found to be more effective than MTX alone, according to a meta-analysis of ten randomized controlled trials (RCTs) [9]; however, there is still a lack of strong evidence regarding the effects of SIN alone in RA therapy.
Modulation of arginine metabolism was found to alter cellular metabolism in bone and to regulate the progression of arthritis [10,11]. Arginine supplementation resulted in decreased osteoclasts numbers in the bone, resulting in less bone damage [12]. SIN has been found to upregulate the gene level of arginase 1, which may promote a decrease in arginine and alleviate RA [13]. Here, we first conducted a 24-week, randomized, placebocontrolled, double-blind clinical trial to evaluate the efficacy and safety of SIN in patients with RA. We also hypothesized that the contribution of SIN to arginine metabolism processes would be detectable in the RA patients and that serum metabolites related to arginine metabolism, such as ornithine, citrulline, and arginine, could act as candidate treatment response biomarkers for SIN in RA.
《2. Methods》
2. Methods
《2.1. Study design》
2.1. Study design
This 24-week, randomized, placebo-controlled, double-blind, and double-imitation clinical study was conducted at the Southwest Hospital of Army Military Medical University, the People’s Hospital of Changshou Chongqing, and the Traditional Chinese Medicine Hospital Dianjiang Chongqing. The study was approved by the Medical Ethics Committee of the Southwest Hospital of Army Military Medical University (2015–65) and registered with the World Health Organization (WHO) clinical trial registry (No. ChiCTR-IPR-16008793). Written informed consent was obtained from all the participants.
《2.2. Participants》
2.2. Participants
Eligible participants were at least 18 years of age with mild to moderate active RA and fulfilled the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) criteria [14]. The patients had mild to moderate active arthritis, which is defined as a 28-joint disease activity score (DAS28) greater than 2.6 and less than or equal to 5.1. The patients had not received any conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), biological DMARDs, SIN preparations, or traditional Chinese medicine for at least one month.
Patients were excluded if they had a serum aspartate transaminase or alanine transaminase concentration greater than 1.5 times the upper limit of normal; a serum creatinine level higher than the upper limit of normal; a white blood cell count below 3.0 × 109 L–1; a hemoglobin concentration of less than 80 g·L–1; or a platelet count of less than 8.0 × 1010 L–1. Patients were also excluded if they had a history of severe, progressive cardiac, hepatic, renal, or mental diseases; other rheumatic autoimmune diseases; any current infection; or any cancer.
《2.3. Randomization and blinding》
2.3. Randomization and blinding
We randomly assigned patients using a randomized-block design with a schedule generated by the Medical Management Center at the World Federation of Chinese Medicine Societies using statistical analysis system (SAS) software. The block size was set as 6, and the patients and investigators were masked to the allocation. Each patient was given a number for the drugs according to the sequence of patient enrollment, and then obtained the drugs from the clinical center based on the drug number. A doubleblind and double-simulation trial was conducted in which the investigators, patients, and statistician were blinded to the interventions.
《2.4. Treatment and outcomes》
2.4. Treatment and outcomes
Eligible patients were assigned (1:1:1) the following treatments: for the SIN group, twice daily controlled-release SIN at 120 mg and MTX imitation at four tablets once a week; for the MTX group, SIN imitation at two tablets twice daily and MTX at 10 mg once a week; or, for the combination group, controlledrelease SIN at 120 mg twice daily and MTX at 10 mg once a week for 24 weeks. SIN was provided by Hunan Zheng Qing Pharmaceutical Group Co., Ltd. (National Medical Products Administration Z20010174) under the name ZQFTN. The purity of the SIN in each ZQFTN pill was greater than 99.7%. The imitation drugs and study drugs were identical in appearance.
Four study visits occurred over 24 weeks, at week 0, week 4, week 12, and week 24. Efficacy, patient-reported outcomes, laboratory assessments, and adverse event assessments were measured at these four time points. We set the primary endpoints as the proportion of patients who achieved a 50% improvement in the (ACR) (ACR50) criteria at week 24 and improvement in the clinical disease activity index (CDAI). Key secondary endpoints were the proportions of participants that achieved 20% (ACR20) or 70% (ACR70) improvement in the ACR criteria over time.
To detect side effects, vital signs detection, physical examinations, and laboratory tests containing routine blood tests, routine urine tests, and blood biochemical tests were conducted at every visit. Electrocardiography and chest X-ray examinations were also performed at the screening visit and at week 24.
《2.5. Sample collection and preparation》
2.5. Sample collection and preparation
Serum samples were collected from the patients at every study visit and were stored in a refrigerator at –80 °C. A sample size of 221 was planned, with 135 recruited patients in three treatment groups at four time points. Aside from the carboxyl-containing metabolites reported in our previous article [15], the other carboxylic acid standards—that is, ornithine and citrulline—were obtained from Sigma-Aldrich Laboratories, Inc. (USA) and J&K Scientific (China). 5-(Diisopropylamino)amylamine (DIAAA), 1-hydroxybenzotriazole hydrate (HOBt), O-(7-azabenzotriazol-1- yl)-N,N,N' ,N' -tetramethyluronium hexafluorophosphate (HATU), and triethylamine (TEA) were also purchased from Sigma-Aldrich Laboratories, Inc. Acetonitrile (ACN, liquid chromatography (LC)/mass spectrometry (MS) grade) and methanol (MeOH, high performance liquid chromatography (HPLC) grade) were obtained from Anaqua Chemicals Supply Inc., Ltd. (USA). Deionized water was prepared using a Millipore water purification system (Millipore Corp, USA). MS-grade formic acid and other chemical reagents were provided by Sigma-Aldrich Laboratories, Inc.
The metabolites were analyzed using our previously developed ultra-high performance liquid chromatography (UHPLC)-quadrupole time-of-flight (TOF) mass spectrometry (UHPLC-Q-TOF/MS) approach [15]. In brief, 50 μL serum samples were precipitated with four volumes of cold MeOH, followed by centrifugation at 13 000 revolutions per minute (rpm) for 5 min at 4 °C. This procedure was repeated twice, and then the supernatants were combined and dried under a nitrogen stream. The residue from the 50 μL serum samples was sequentially mixed with 5 μL of 20 mmol·L–1 HOBt in dimethyl sulfoxide (DMSO), 5 μL of 100 mmol·L–1 DIAAA–TEA in DMSO containing 200 mmol·L–1 TEA, and 5 μL of 20 mmol·L–1 HATU in DMSO, followed by 1 min of incubation at room temperature. Finally, 35 μL ACN was added to make up a final volume of 50 μL, and 1 μL was directly injected into the UHPLC-Q-TOF/MS.
《2.6. UHPLC-Q-TOF/MS analysis》
2.6. UHPLC-Q-TOF/MS analysis
An Agilent (USA) 1290 Infinity LC system (UHPLC) was employed for chromatographic analysis, and a Waters ACQUITY UPLC HSS T3 column (2.1 mm × 100 mm, 1.8 μm) was used to separate the metabolites. The column temperature was maintained at 30 °C, and the autosampler was set at 4 °C. The flow rate was 0.3 mL·min–1 . The injection volume was 1 μL. Mobile phases A and B were water containing 0.1% formic acid and ACN containing 0.1% formic acid, respectively, and the gradient was set as follows: 0–0.5 min, 2%–5% B and the remaining 98%–95% A; 0.5–2.5 min, 5%–6% B; 2.5–4.5 min, 6%–7% B; 4.5–5.5 min, 7%–7.3% B; 5.5–7.5 min, 7.3%–7.8% B; 7.5–11 min, 7.8%–9% B; 11–13 min, 9%–14% B; 13–17 min, 14%–23% B; 17–19 min, 23%–25% B; 19–26.5 min, 25%–35% B; 26.5–28.5 min, 35%–47% B; 28.5–33 min, 47%–60% B; 33–35 min, 60%–95% B; 35–37.9 min, 95% B; and 38 min, 2% B.
MS was performed on an Agilent 6545 accurate-mass Q-TOF/MS system equipped with an electrospray ionization source (ESI) in positive (POS) ion mode. The MS parameters were set as follows: dry gas temperature, 300 °C; dry gas flow, 11 L·min–1 ; sheath gas temperature, 325 °C; sheath gas flow, 11 L·min–1 ; nebulizer pressure, 35 psig (1 psig = 6894.76 Pa); capillary voltage, 3500 V; and nozzle voltage, 500 V. The mass spectra m/z range for the derivatized samples was 200–1000 Da. Accurate mass measurements were obtained using a low flow of the TOF reference mixture containing the internal reference mass at m/z 922.0098 (C18H18F24N3O6P3).
《2.7. Metabolite identification》
2.7. Metabolite identification
MassHunter qualitative analysis software (Agilent Technologies, Inc., China) was applied for raw data mining. Metabolites with relevant standards were identified by comparison with the standards. Others were elucidated through tandem MS (MS/MS) spectra and the Lipid Maps metabolites database↑ , the Human Metabolome Database , and the METLIN Metabolite and Chemical Entity Database↑↑. The statistical analysis was performed using GraphPad Prism version 5.0 (GraphPad Software, USA).
, and the METLIN Metabolite and Chemical Entity Database↑↑. The statistical analysis was performed using GraphPad Prism version 5.0 (GraphPad Software, USA).
↑ http://www.lipidmaps.org/.
 http://www.hmdb.ca/.
http://www.hmdb.ca/.
↑↑ https://metlin.scripps.edu/index.php.
《2.8. Statistical analysis》
2.8. Statistical analysis
The efficacy and safety of the SIN, MTX, and SIN + MTX combination treatments were assessed. In the intention-to-treat (ITT) analysis, data were collected from the last observation, while in the per-protocol (PP) analysis, data were calculated by using the data from those who finished the 24-week observation period. For the primary efficacy endpoints, dichotomous variables were analyzed using Pearson’s χ2 test. The CDAI is a composite measure of disease activity and does not include ESR or CRP; thus, it was suitable for comparing the new biomarker with current markers, such as ESR or CRP. The secondary efficacy endpoints were assessed by means of a one-way repeated measures analysis of variance (ANOVA) of the mean values from the baseline to weeks 4, 12, and 24 for each group.
The significance of the metabolites in group discrimination was further measured by Student’s t-test via multivariate approaches. A P value lower than 0.05 was considered to be significant. Changes in the identified inflammatory biomarker levels according to the different disease activities were compared between groups using the Mann–Whitney U test. We also compared the levels of ornithine, ESR, and CRP at weeks 4, 12, and 24 by means of the Wilcoxon signed-rank sum test. Sensitivity to change after 4, 12, and 24 weeks was assessed by standardized response means (SRMs; mean change divided by the standard deviation (SD) of the change scores).
《3. Results》
3. Results
In total, 135 RA patients classified according to the 2010 ACR/ EULAR criteria were randomly assigned to SIN or MTX monotherapy or to SIN + MTX combination therapy [16]. Ultimately, 38, 39, and 36 patients treated with SIN, MTX, and SIN + MTX, respectively, were analyzed in the ITT cohort (Fig. 1). The baseline data were generally similar among the three treatment groups (Table S1 in Appendix A).
《Fig. 1》

Fig. 1. Flowchart of the RCT protocol. Bid: twice daily; qw: once a week.
At week 24, 52.63% of the patients in the SIN-treated group, 48.72% of the patients in the MTX-treated group, and 58.33% of the patients in the SIN + MTX-treated group achieved ACR50 (Fig. 2(a)) in the ITT analysis. The other primary endpoint, the CDAI scores, were significantly decreased in the SIN, MTX, and SIN + MTX treatment groups over the treatment time (P < 0.0001 for each group). Moreover, the CDAI scores at week 24 in the SIN group, as well as those in the SIN + MTX group, were significantly lower than those in the MTX group (SIN vs MTX: P < 0.05; SIN + MTX vs MTX: P < 0.01) (Fig. 2(b)). The key secondary end-points of ACR20 and ACR70 were achieved and were comparable among the three treatment groups at week 24, although the onset time of the SIN group seemed to be a little slower than that of the MTX group (Figs. 2(c) and (d)). There were also significant improvements in each individual component of the ACR score (tender joint count (TJC) 28, swollen joint count (SJC) 28, health assessment questionnaire disability index (HAQ-DI), patient’s global assessment of disease activity (PaGADA), physician’s global assessment of disease activity (PhGADA), CRP, and ESR) (Table 1). The descent rate of rheumatoid factor (RF) and cyclic citrullinated peptides (CCPs) showed a faster trend in the SIN and SIN + MTX groups than in the MTX group (Table 1). In the PP analysis, the SIN + MTXtreated group achieved a significantly higher ACR50 response rate (86.96%) in comparison with the MTX group (55.88%), while being comparable with the SIN group (72%); this finding indicates that a combination treatment with SIN and MTX may be a preferable choice for patients that are non-responsive to MTX (Fig. S1 in Appendix A).
《Fig. 2》
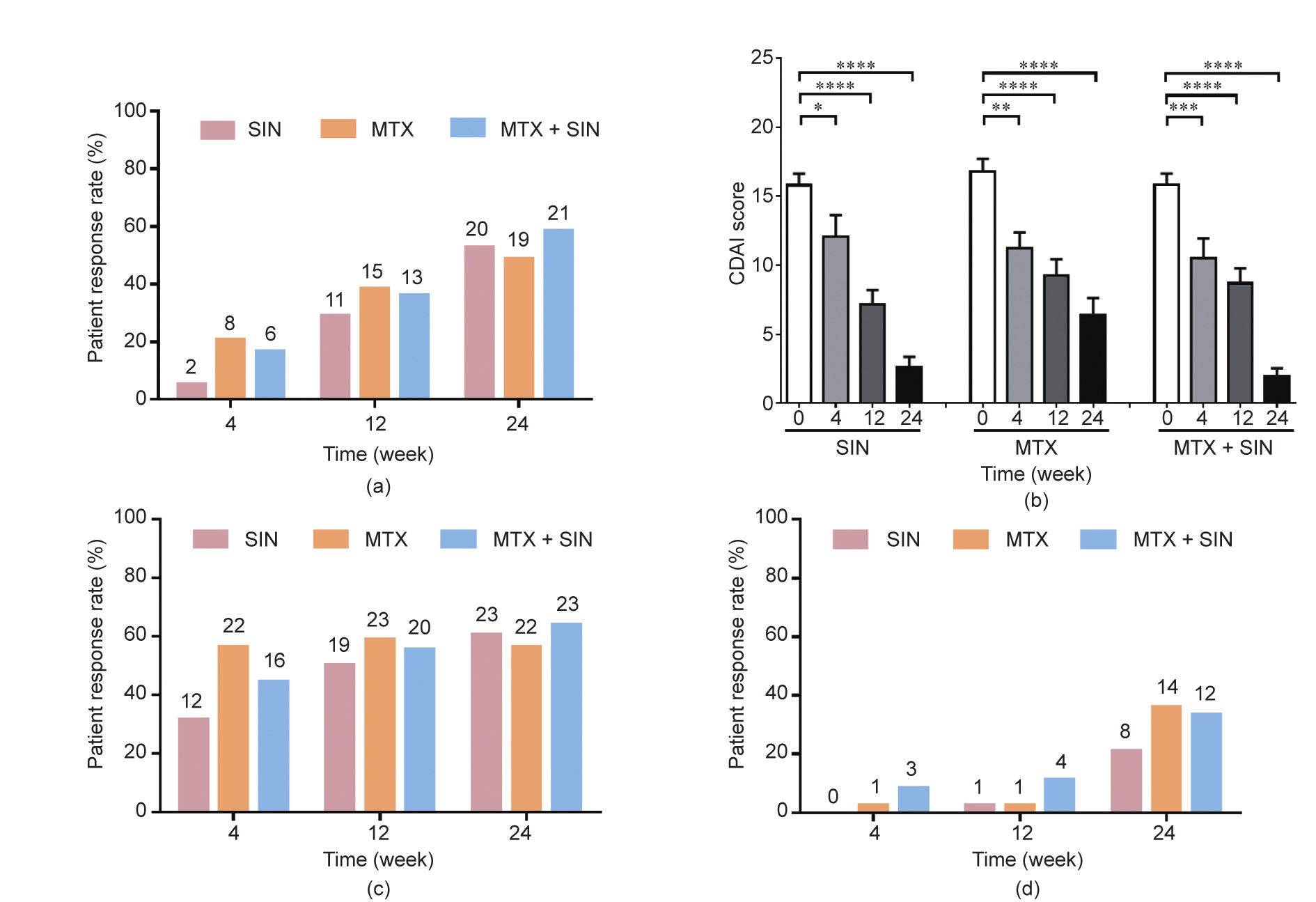
Fig. 2. Primary and secondary efficacy measures over time. (a) ACR50 response rate of RA patients in the three treatment groups; (b) changes in the CDAI at the four follow-up time points in the three treatment groups; (c) ACR20 response rate; (d) ACR70 response rate. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
《Table 1》
Table 1 Clinical and laboratory measures of the three treatment groups of RA patients at each visit.
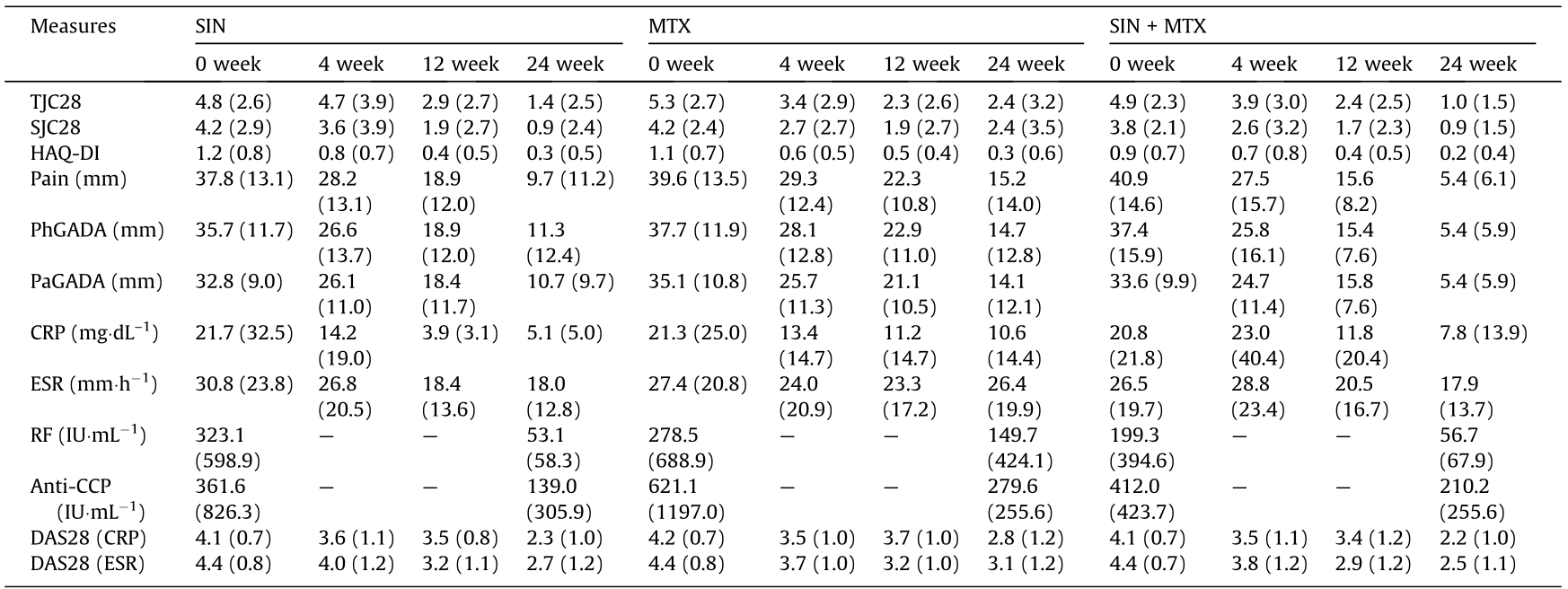
Data are presented as the mean (SD).
RF was measured by immunonephelometry with a cutoff value of 20 IU·mL–1 ; anti-CCP was measured using a commercially available second-generation enzyme-linked immunosorbent assay (ELISA) kit (Abbott, USA) with a cutoff value of 25 IU·mL–1 .
Hepatic and gastrointestinal disorders were the main adverse events reported in our study. It was notable that the ratio of patients suffering from hepatic disorder in the SIN group (1/38) was much lower than that in the MTX (10/39) and SIN + MTX (8/36) groups (Table S2 in Appendix A). A total of 14 patients in the MTX group suffered from gastrointestinal disorder, which was a much higher proportion than in the SIN + MTX and SIN groups. The main side effects for SIN were rash and skin itch.
The serum levels of ornithine, citrulline, and arginine, which were obtained through our previously developed UHPLC-Q-TOF/ MS approach [15], were significantly correlated with the clinical indices (Table S3 in Appendix A), and the ornithine levels decreased after 4, 12, and 24 weeks of treatment (Fig. 3(a)). Patients treated with SIN, MTX, and SIN + MTX revealed a significant reduction in ornithine levels, along with relief from the disease (Fig. 3(b)). The SRM for ornithine, which reflects the degree of responsiveness to disease activity, was comparable to the CRP levels and higher than the ESR (Fig. S2 in Appendix A).
The CDAI has been preferred as the most suitable composite measure of disease activity for comparisons among ornithine, ESR, and CRP [17]. To determine whether ornithine reflects the CDAI level, we divided the samples into four subgroups according to the CDAI: high, moderate, or low disease activity, or remission. A significantly higher level of ornithine was detected in patients with high and moderate CDAI scores than in patients with low activity or remission (Fig. 3(c)). A similar trend was found for CRP levels and ESR (Fig. S3 in Appendix A). These results suggest that ornithine can be used to evaluate disease activity. Further analysis found that 39.1% of the patients who achieved ACR50 at week 24 showed an ornithine level above the median at week 0, while only 20.0% of the non-responders revealed an ornithine level above the median. Moreover, we used the correlation-based feature subset selection method (CfsSubsetEval) in Waikato Environment for Knowledge Analysis (WEKA) to detect the predictive capacity of ornithine in achieving ACR50 after 24 weeks of treatment. According to the receiver operating characteristic (ROC) analysis, the area under the curve (AUC) was 0.717, with a sensitivity value of 80% and a specificity of 55.6% (Fig. 3(d)), which supports the conclusion that the serum ornithine level may reflect therapeutic responses.
《Fig. 3》

Fig. 3. The serum level of ornithine functions as a biomarker of inflammation. (a) The normalized peak area of ornithine in all three groups of patients after 4, 12, and 24 weeks of treatment; (b) the normalized peak area of ornithine in the patients in the SIN, MTX, or SIN + MTX groups, respectively; (c) the normalized peak area of ornithine in patients with different CDAI levels: high disease activity (CDAI > 22), moderate disease activity (22 > CDAI > 10), low disease activity (10 > CDAI > 2.8), or remission (CDAI < 2.8); (d) the capacity of ornithine for predicting the ability of patients to achieve ACR50 after the 24-week treatment. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
《4. Discussion》
4. Discussion
In this report, we first assessed the efficacy and safety of SIN for the treatment of patients with RA by conducting a prospective, multicenter, double-blind trial. We then further identified the correlations between the alleviation of RA and ornithine, which may indicate potential therapeutic mechanisms of SIN on RA.
SIN has usually been used as a complementary treatment with csDMARDs in RA therapy [8]; however, there is little evidence to show that SIN alone has positive effects against RA. This was the first clinical trial focusing on the effects of SIN monotherapy. We observed comparable effects between SIN treatment from weeks 4 through 24 and treatment with the positive control, MTX, according to both the primary endpoints (ACR 50 and CDAI) and the secondary key endpoints. These results indicate that SIN probably acts as a natural anti-rheumatic drug in RA therapy. Moreover, the results of the PP analysis revealed that the efficacy in the SIN + MTX combination therapy group was better than that in the MTX group; the combination therapy also had a lower incidence of adverse events, indicating that SIN + MTX combination therapy might be preferable to MTX for RA patients with mild to moderate active arthritis.
According to the literature, the amino acids related to arginine metabolism, including citrulline, ornithine, and arginine, were found to be elevated in RA patients in comparison with healthy controls [18,19]; the circulating levels of ornithine were also found to be elevated in patients with musculoskeletal pain [20]. In the current study, we also found close correlations between ornithine and disease activity, although the possible mechanisms behind this correlation remain unknown. Ornithine derives from arginine and can be metabolized in citrulline, collagen, or glutamate [21,22]. Citrulline is a protein following posttranslational modification by peptidyl arginine deiminase (PAD), which is strongly implicated in RA at both the genetic and cellular levels; its inhibitors have demonstrated therapeutic efficacy in inflammatory arthritis [23]. Citrulline also acts as an antibody to anti-CCP [24], so it has been speculated that elevated ornithine levels may promote the generation of anti-CCPs. In addition, ornithine is important in collagen synthesis and wound repair. RA can result in the degradation of cartilage and bone with a chronic degenerative state, and type II collagen is the major and specific molecule in articular cartilage [25]. Thus, it is probable that elevated levels of ornithine are associated with a compensatory or repair mechanism for bone damage. Moreover, glutamate is relevant in RA, and its concentrations in synovial fluid were reported to increase more than 50 folds in patients with RA [26], which was found to stimulate tumor necrosis factor-α (TNF-α) expression [27]. Ornithine is a precursor of glutamate, so elevated ornithine levels may affect the expression of TNF-α via the effects of glutamate.
SIN can upregulate the gene level of arginase 1 [13], which may promote a decrease in arginine and further down-regulate ornithine. Our study is the first to detect long-term alterations in ornithine levels over 24 weeks of treatment with SIN. The serum levels of ornithine fluctuated with patients’ CDAI scores and were significantly higher in patients with high disease activity than in those with low activity or remission. The level of ornithine at week 0 also had the potential to predict patients’ therapeutic response at week 24, regardless of whether the patients were in the SIN, MTX, or combination SIN + MTX groups; this finding indicates that SIN may affect the development of RA by regulating ornithine.
The current study has some limitations. The sample size of this study was relatively small. Center labs were not adopted to avoid variations among different hospitals. Furthermore, the radiological score for the evaluation of bone destruction in X-ray or magnetic resonance imaging (MRI) was not evaluated.
In summary, this study provides clinical evidence of the efficacy of SIN therapy on RA. This study showed that SIN has a therapeutic efficacy comparable to that of MTX, but with much fewer adverse effects. This is also the first report on the correlation between ornithine level and disease alleviation in RA patients, which suggests the potential therapeutic mechanisms of SIN against RA. Further intensive analysis is required to confirm this correlation.
《Acknowledgments》
Acknowledgments
The authors thank Dr. Shumei Yan from the People’s Hospital of Changshou Chongqing, Dr. Yong Wang from the Southwest Hospital of Army Military Medical University, and Dr. Xiaofen Jiang from the Traditional Chinese Medicine Hospital Dianjiang Chongqing, for their valuable help in undertaking this study. The authors also thank Fei-chi Wu and Ping Qiu from Hunan Zheng Qing Pharmaceutical Group Co., Ltd. for providing part of the funding and the study drug (ZQFTN) for this research.
This work was financially supported by grants from the Macao Science and Technology Development Fund (0032/2018/AFJ, 0003/2019/AKP, and 0010/2020/A1) and the Key Program in Emerging Industry of the Hunan Department of Science & Technology (2014GK1058). This work was also funded by Dr. Neher’s Biophysics Laboratory for Innovative Drug Discovery (001/2020/ALC) supported by the Macao Science and Technology Development Fund.
《Authors’ contributions》
Authors’ contributions
Conceptualization, L Liu and YF Fang; clinical observation and sample collection, Y Shi, QH Zou, HG Li, and WF Leng; methodology, Y Shi, JL Wu, Y Wang, XQ Bian and CJ Wang; data analysis, HD Pan and YF Wang; writing, HD Pan; manuscript revision, L Liu, HD Pan, and YF Fang; supervision, L Liu and YF Fang; and funding acquisition, L Liu and YF Fang.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Ying Shi, Hu-Dan Pan, Jian-Lin Wu, Qing-Hua Zou, Xin-Yi Xie, Hong-Gang Li, Hua Zhou, Xi-Qing Bian, Wen-Fei Leng, Can-Jian Wang, Ya-Feng Wang, Yong-Fei Fang, and Liang Liu declare that they have no conflict of interest or financial conflicts to disclose.
《Appendix A. Supplementary data》
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eng.2021.04.014.














 京公网安备 11010502051620号
京公网安备 11010502051620号




