《1. Introduction》
1. Introduction
As many as 50% of infertile couples have infertility due to male factors. Causes of infertility in men include congenital or acquired urogenital abnormalities, endocrine disorders, and genetic and immunological problems; however, most cases are idiopathic, with no definite causes that can be identified by clinical examination [1]. Along with infertility, low sperm concentration, poor sperm motility, abnormal morphology, or a combination of these are often presented in idiopathic male infertility [2].
Medicines and supplements has been widely used to treat idiopathic male infertility [3]. Such medicines—which are mainly hormones and hormonal modulators—exert their effects via the hypothalamic–pituitary–testicular axis to improve sperm quality, eventually allowing the couple to become pregnant. Of these, follicle-stimulating hormone (FSH) and selective estrogen receptor modulator (SERM; mainly clomiphene or tamoxifen) are frequently used [4], despite not being approved by the US Food and Drug Administration (FDA). A meta-analysis has indicated that SERM, compared with a placebo or being untreated, significantly increased spontaneous pregnancy rate (SPR), as well as sperm concentration and progressive motility [5]. To date, the benefits of FSH administration for idiopathic male infertility remain unclear. Furthermore, the optimal treatment for idiopathic male infertility is still unknown, although it is likely to be FSH or SERM, among others.
Aside from medicines, another regimen that is extensively used involves supplements, including zinc, selenium, vitamin C or E, and carnitine. Most of these supplements act as antioxidants to protect sperm from damage from reactive oxygen species (ROS) [6]. L–carnitine, for example, is a quaternary amine found in the epididymis and spermatozoa [7] that is frequently prescribed for infertile men. A meta-analysis has indicated that L–carnitine, compared with a placebo or vitamins, significantly increased not only sperm quality but also SPR [8]. Another supplement-related mechanism involves the regulation of mitochondrial bioenergetics; for example, coenzyme Q10 (CoQ10) is a component of the mitochondrial respiratory chain that is involved in energy production, which provides energy for sperm maturation and motility [9]. Previous studies have shown that CoQ10, compared with a placebo, significantly improved sperm concentration and motility [10,11], although the effectiveness of CoQ10 on fecundity outcomes is not validated. Moreover, there is a debate regarding whether supplements plus medicines achieve better benefits than either supplements or medicines alone.
Although many trials on male infertility have been conducted, it is difficult to determine the optimal treatment, as direct evidence from trials comparing certain medications is lacking. A network meta-analysis (NMA) is designed to compare multiple treatments in one statistical model [12–14] and provides a hierarchy of the efficacies of these treatments to guide clinical practice [15,16], according to a rigorous methodology. In this study, therefore, we performed a systematic review update and NMAs to compare the effectiveness of different medicines and supplements on live birth rate, SPR, and semen parameters in idiopathic male infertility.
《2. Materials and methods》
2. Materials and methods
《2.1. Protocol and registration》
2.1. Protocol and registration
The protocol was registered (CRD42020158348) in the PROSPERO registry↑ . We followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) extension statement for NMAs [17].
↑ http://www.crd.york.ac.uk/PROSPERO.
《2.2. Literature search》
2.2. Literature search
We searched electronic databases, including Medline, Excerpta Medica Database (EMBASE), Ovid, and China National Knowledge Infrastructure (CNKI), for the period from January 1990 to June 2021. The keyword-based searches were applied for studies on idiopathic male infertility, medicine, and supplements, along with a specific filter for clinical trials using the following keywords in combination with both medical subject heading terms and text words: male infertility, oligospermia, asthenozoospermia, oligoasthenozoospermia (OA), oligoasthenoteratozoospermia (OAT), diet therapy, trace element, supplementation, carnitine, vitamin, antioxidant, ubiquinone, amino acid, fatty acid, drug therapy, SERM, aromatase inhibitors, androgen, steroid, gonadotropin, and randomized controlled trial (RCT; Table S1 in Appendix A). No language restrictions were applied, and conference abstracts were excluded from the analysis.
《2.3. Study selection》
2.3. Study selection
Only published RCTs were included in the data extraction and analysis, including RCTs on:①idiopathic infertilemales with abnormal semen parameters, according to the World Health Organization (WHO) criteria [18], including teratozoospermia (percentage of normal morphology < 4%), oligozoospermia (spermatozoa count < 1.5 × 107 mL–1 ), asthenozoospermia (percentage of motile spermatozoa < 40%), and two or more abnormalities, without any known cause of impaired spermatogenesis or hormonal abnormality; ② a comparison of medicine, supplements, or a combination of one or more of these with a placebo, no treatment, or others; and ③ measured pregnancy outcomes (including spontaneous biochemical/ clinical pregnancy and live birth), sperm parameters (including concentration, motility, and normal morphology), and/or side effects. The medicines studied included sex hormones (e.g., recombinant or purified FSH, gonadotropin, and androgen such as testosterone and its derivatives), SERM (tamoxifen and clomiphene), aromatase inhibitors (e.g., letrozole), or other medicines. Supplements included trace elements (e.g., zinc and selenium), vitamins (e.g., vitamins C, E, D3, and folic acid), energetic supplements (e.g., carnitine, CoQ10, and fatty acids), or others, such as docosahexaenoic acid (DHA) and probiotics. Studies were excluded if they were a quasi-RCT or other study design, duplicated publication, or overlapping participants by the same authors. Two independent reviewers conducted the literature search and study selection. Any disagreements were resolved by consensus after discussion.
《2.4. Data extraction and outcomes》
2.4. Data extraction and outcomes
Two reviewers independently assessed the full texts and extracted data from the included original papers using a specifically designed form that captured information on the study design, trial setting, patient characteristics (inclusion and exclusion criteria), sample sizes, details of treatment (intervention, comparison, and duration), outcomes (live birth, SPR, and sperm parameters), and side effects. Discrepancies were resolved by discussion or consultation with a third reviewer.
The primary outcomes included live birth and SPR. Live birth was defined as delivery of a viable fetus after 28 weeks of gestation. Pregnancy included biochemical and clinical pregnancy and was defined as any positive serum human chorionic gonadotropin (hCG) test or an intrauterine pregnancy with fetal heart pulsation as detected by transvaginal ultrasound, respectively. The secondary outcomes were sperm parameters, including sperm concentration, progressive motility, and morphology, and side effects.
《2.5. Risk of bias and quality of evidence assessment》
2.5. Risk of bias and quality of evidence assessment
Methodological quality was assessed independently by two reviewers using the Cochrane risk-of-bias tool for RCTs [19]. Included RCTs were classified into one of three categories: low risk, high risk, or unclear risk. The grades of recommendation, assessment, development, and evaluation (GRADE) system adapted to an NMA was employed to grade the quality of the evidence into four levels: high, moderate, low, and very low [20]. Finally, since different comparisons might be characterized by a different risk of bias, the relative contribution of each piece of direct evidence was properly accounted for, using the data from the network contribution matrix [20].
《2.6. Data synthesis and statistical analysis》
2.6. Data synthesis and statistical analysis
We conducted an NMA to explicitly address any difference on therapeutic regimens, combining both direct and indirect estimates of relative treatment effect into a single analysis, which is thereby less prone to bias. All NMAs were conducted within a random-effects multiple regression model using the ‘‘network” and ‘‘mvmeta” packages in Stata software (version 15.0; Stata Corp LP, USA) [21,22]. Prior to conducting the NMA, inconsistency was assessed by using both local and global methods in Stata as appropriate, and by calculating the I2 for network heterogeneity and inconsistency [21,23]. No significant inconsistency was found upon assessing all outcomes in the NMA. Studies with 0 or 100% events in all interventions were excluded from the analysis due to a lack of information regarding relative effects. For studies with zero events in one arm only, we added a continuity correction of 0.5 to each cell. We presented a summary of the treatment effects as relative risk (RR) or mean difference (MD) with 95% confidence intervals (95%CI) to facilitate the interpretation of the results in term of the magnitude of heterogeneity. We applied a comparison-adjusted funnel plot to assess small-study effects in the network and the surface under the cumulative ranking curve (SUCRA) to provide a summary statistic for the cumulative ranking of treatments [21]. The SUCRA is a percentage of the effectiveness of every intervention relative to an imaginary treatment that is always the best without uncertainty. The higher the SUCRA value, the higher the likelihood of effective treatment [24]. We also performed a subgroup and sensitive analysis according to the risk of bias, abstinent time, and type of sperm abnormality, respectively. Statistical analysis and graph generation were prepared by Stata software. Risk of bias was assessed using the dedicated Cochrane tool of Review Manager (version 5.3; The Cochrane Collaboration, Denmark). All statistical tests were two-sided, and a p < 0.05 was defined as statistically significant.
《3. Results》
3. Results
《3.1. Characteristics of the included studies》
3.1. Characteristics of the included studies
The literature search yielded 6675 publications. After screening the titles and abstracts, 293 studies were potentially considered eligible for inclusion in the review and were then further evaluated by retrieving the full text. Sixty-five studies (7541 men) were eventually included in the current study. A flow chart describing the literature selection process is presented in Fig. 1.
《Fig. 1》
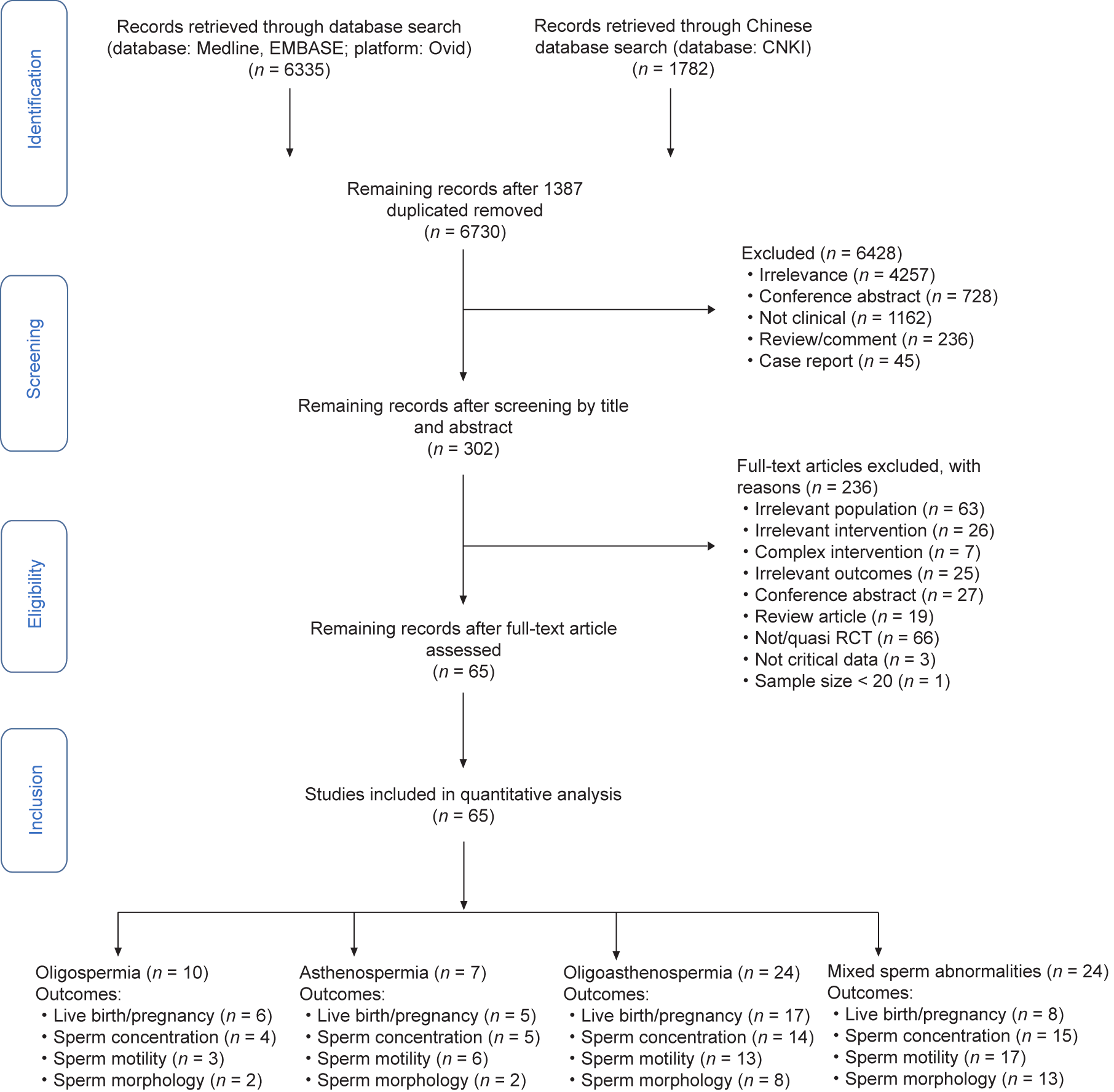
Fig. 1. PRISMA flow chart.
The included studies were conducted in various countries and published in English, except for ten studies (15.4%) that were published in Chinese. The characteristics of the included studies are presented in Table S2 in Appendix A. All RCTs declared that the participants were diagnosed with idiopathic male infertility without hormone abnormality. Of the RCTs, ten studied oligozoospermia [25–34], seven studied asthenozoospermia [35–41], 24 studied OA [42–65], and 24 studied more than two types of sperm abnormality [66–89]. Five RCTs did not provide basal hormone concentrations, and 46 RCTs reported 2–7 days of abstinence time (AT) prior semen analysis before and after treatment. All trials measured sperm parameters, only three RCTs reported live birth rates, and 36 RCTs reported SPR. Only SPR and sperm parameters could be analyzed by the NMA. The studied medicines included SERM (12 RCTs, 18.5%), FSH (11 RCTs, 16.9%), androgen (4 RCTs, 6.0%), pentoxifylline (3 RCTs, 4.6%), gonadotropin-releasing hormone (GnRH) (2 RCTs, 3.1%), and others (only one study on each of the other medicines, 1.5%) including hCG, kallikrein, and indomethacin. The studied supplements included carnitine (12 RCTs, 18.5%), vitamin C or E (7 RCTs, 10.8%), CoQ10 (9 RCTs, 13.8%), folic acid (2 RCTs, 3.1%), acetylcysteine (2 RCTs, 3.1%), zinc (2 RCTs, 3.1%), and others (only one study on each of the other supplements, 1.5%), including omega-3 fatty acids, selenium, resveratrol, and lipoic acid. The studied medicines combined with supplements included SERM plus vitamins (4 RCTs, 6.2%), carnitine plus vitamins (3 RCTs, 4.6%), SERM plus androgen (2 RCTs, 3.1%), selenium plus vitamins (2 RCTs, 3.1%), and others (only one study on each combination of medicines and supplements, 1.5%), including SERM plus carnitine, SERM plus CoQ10, SERM plus kallikrein, pentoxifylline plus carnitine, and selenium plus acetylcysteine. Forty-six (70.8%) RCTs used a placebo and six (9.2%) RCTs used nontreatment as the control; the remaining studies used other treatments as the control. The length of treatment was commonly 3– 6 months. The dose of FSH was 75–300 international units (IU) on alternate days; that of SERM was 20–50 mg·d–1; that of carnitine was 1–2 g·d–1; that of CoQ10 was 30–200 mg·d–1; and that of vitamin C or E was 200–400 mg·d–1.
《3.2. Quality-of-evidence assessment》
3.2. Quality-of-evidence assessment
The methodological quality of most RCTs was moderate (70.8%); only 11 (16.9%) trials had a high risk of bias on random sequence generation and 12 (18.5%) trials had a high risk of bias on allocation concealment. The risk-of-bias assessment of the individual studies is presented in detail in Fig. S1 in Appendix A. The quality of evidence was mostly moderate to low on different outcomes, as measured using the GRADE system (Table S2). According to the risk-ofbias assessment, numbers of studies had methodological issues (i.e., study limitations); the grade of quality was downgraded mainly due to study limitations, indirectness, and imprecision.
3.2.1. Pregnancy rate
Among 36 RCTs (3439 men), 32 were two-arm and four were four-arm RCTs. The network geometry is presented in Fig. 2. The NMA indicated that, compared with a placebo or being untreated, FSH (RR = 2.2, 95%CI, 1.4–3.5), hCG (RR = 2.6, 95%CI, 1.5–4.5), SERM (RR = 2.1, 95%CI, 1.2–3.5), carnitine (RR = 1.9, 95%CI, 1.1–3.3), carnitine plus vitamin C or E (RR = 3.7, 95%CI, 1.6–8.5), carnitine plus CoQ10 (RR = 3.3, 95%CI, 1.6–6.8), SERM plus androgen (RR = 3.2, 95%CI, 1.8–5.9), SERM plus vitamins (RR = 2.2, 95%CI, 1.1–4.6), and SERM plus carnitine (RR = 2.3, 95%CI, 1.2–4.3) resulted in a significantly higher SPR. After excluding RCTs with a high risk of bias or without AT prior to sperm analysis, the effects of FSH, hCG, carnitine, SERM plus androgen, and carnitine plus CoQ10 were still significant, while the effects of SERM, SERM plus vitamins, and carnitine plus vitamins were not (Fig. 3). The SUCRA values evaluated for each intervention for overall, excluded RCTs with a high risk of bias, and excluded RCTs without AT prior to sperm analysis are presented in Fig. 4. The optimal intervention for achieving successful pregnancy was carnitine plus vitamins, regardless of the risk bias of the study or without AT prior to sperm analysis (Table 1 [25–28,30–44,46–61,63–81,83,84,86–89]). The funnel plot indicated a lack of small-study effects for SPR (Fig. 5).
《Fig. 2》
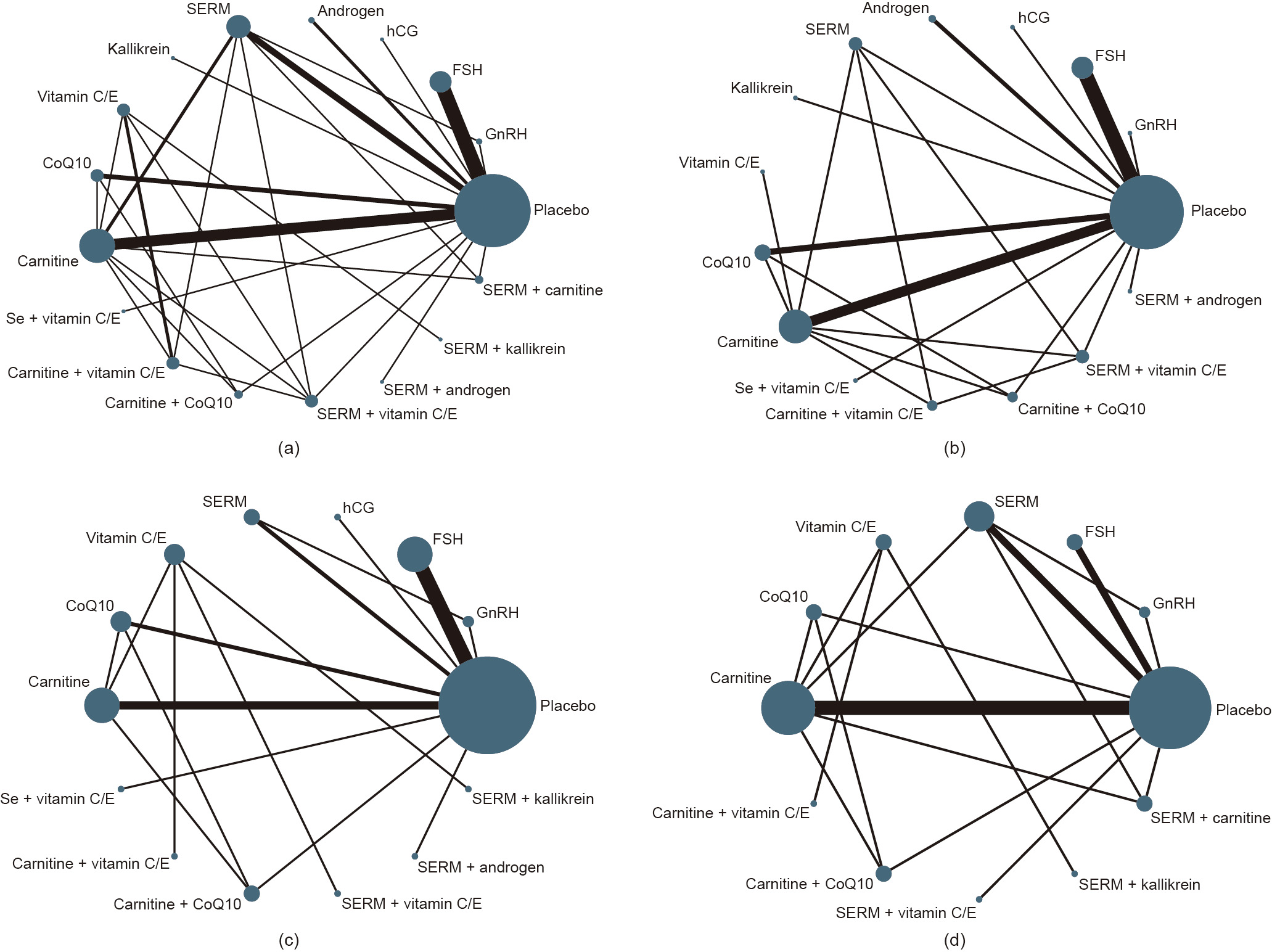
Fig. 2. Network maps of interventions for SPR. (a) Network map with overall interventions; (b) network map with interventions, excluding trials at high risk of bias; (c) network map with interventions, excluding trials without AT prior to sperm analysis; (d) network map with interventions for studying OA. Androgen included testosterone and its derivate; SERM included clomiphene or tamoxifen; Se refers to selenium.
《Fig. 3》
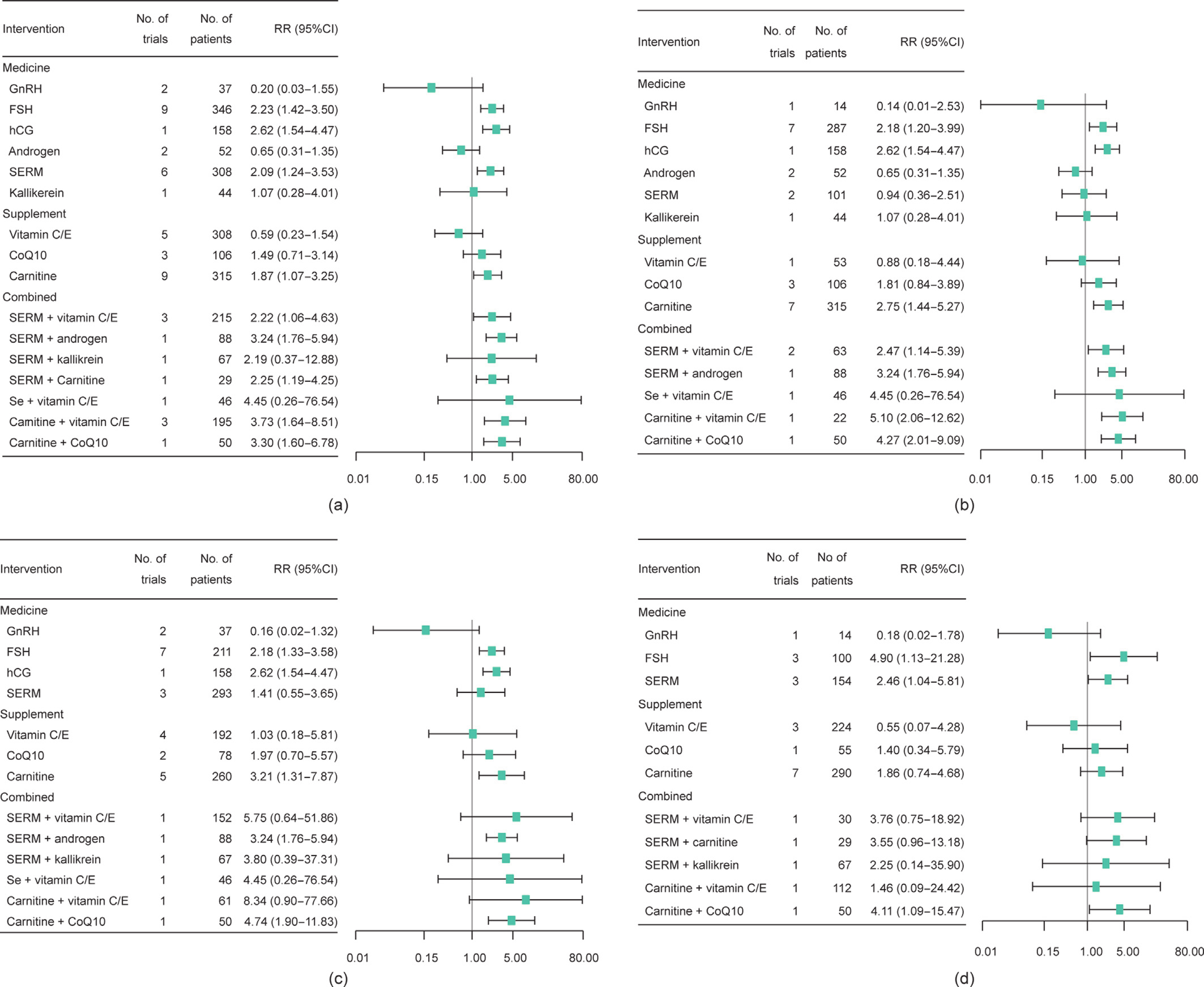
Fig. 3. Forest plots for the estimation of interventions on SPR compared with a placebo or being untreated. (a) Forest plot for estimation of interventions on pregnancy compared with a placebo or being untreated for overall; (b) forest plot for estimation of interventions on pregnancy compared with a placebo or being untreated, excluding trials at high risk of bias; (c) forest plot for estimation of interventions on pregnancy compared with a placebo or being untreated, excluding trials without AT prior to sperm analysis; (d) forest plot for estimation of interventions on pregnancy compared with a placebo or being untreated for studying OA.
《Fig. 4》
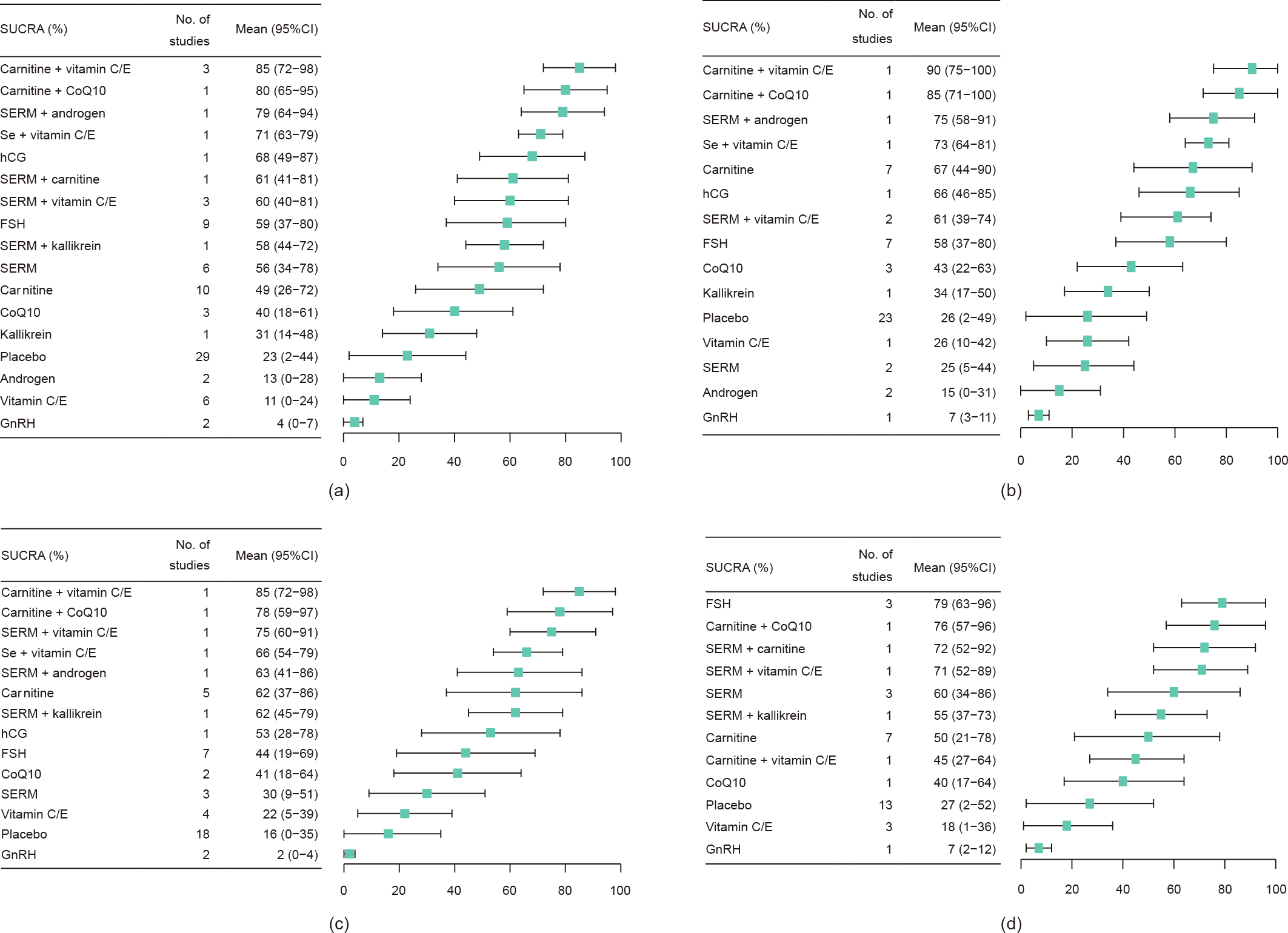
Fig. 4. SUCRA of interventions on SPR compared with a placebo or being untreated. (a) SUCRA of interventions for overall compared with a placebo or being untreated; (b) SUCRA of interventions compared with a placebo or being untreated, excluding trials at high risk of bias; (c) SUCRA of interventions compared with a placebo or being untreated, excluding trials without AT prior to sperm analysis; (d) SUCRA of interventions for studying OA compared with a placebo or being untreated.
《Table 1》
Table 1 The best interventions for different outcomes.

《Fig. 5》
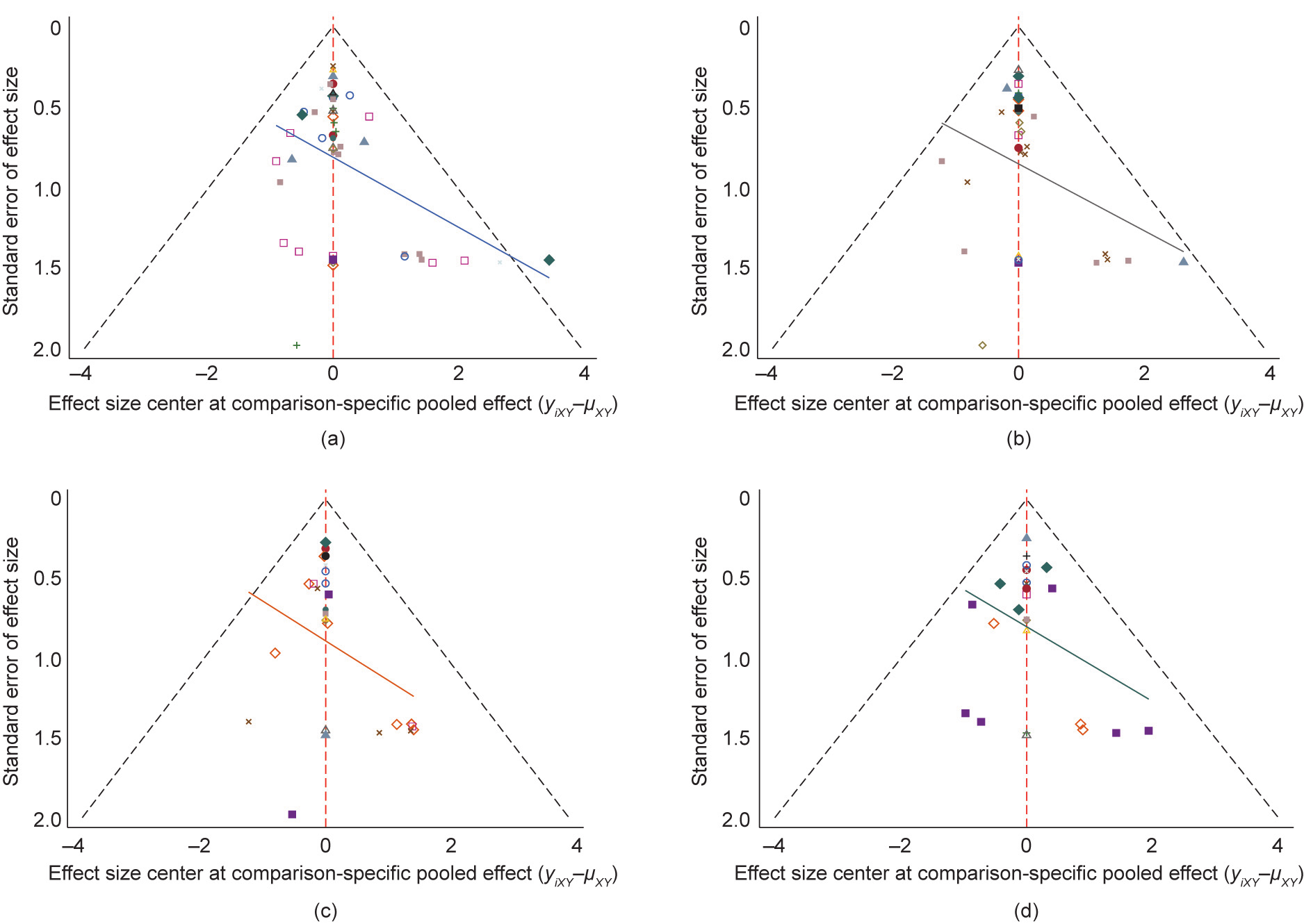
Fig. 5. Funnel plots for SPR. (a) Funnel plot for estimation of reporting bias and between study heterogeneity for overall; (b) funnel plot for estimation of reporting bias and between study heterogeneity, excluding trials at high risk of bias; (c) funnel plot for estimation of reporting bias and between study heterogeneity, excluding trials without AT prior to sperm analysis; (d) funnel plot for estimation of reporting bias and between study heterogeneity studying OA. Different colors correspond to different comparison.
3.2.2. Sperm concentrations
Thirty-seven RCTs (4084 men) were included in the NMA; 32 were two-arm, three were three-arm, and two were four-arm trials. The network geometry is presented in Fig. S2 in Appendix A. Compared with a placebo or being untreated, GnRH (MD = 6.1 × 106 mL–1 , 95%CI, 2.2 × 106 –9.9 × 106 ), SERM (MD = 6.1 × 106 mL–1 , 95%CI, 0.4 × 106 –11.8 × 106 ), pentoxifylline (MD = 10.0 × 106 mL–1 , 95%CI, 0.6 × 106 –19.5 × 106 ), fatty acids (MD = 12.5 × 106 mL–1 , 95%CI, 3.1 × 106 –22.0 × 106 ), SERM plus vitamin C or E (MD = 9.0 × 106 mL–1 , 95%CI, 2.4 × 106 –15.6 × 106 ), and SERM plus CoQ10 (MD = 10.6 × 106 mL–1 , 95%CI, 1.5 × 106 –19.7 × 106 ) resulted in significantly higher sperm concentration. After excluding RCTs with a high risk of bias or with AT prior to sperm analysis, fatty acids were still significant (Fig. S3 in Appendix A). The SUCRA values evaluated for each intervention for overall, excluded RCTs at a high risk of bias, and excluded RCTs without AT are presented in Fig. S4 in Appendix A. The most optimal intervention to improve sperm concentration was fatty acids, regardless of the risk bias of the study. The funnel plot indicated that there were moderate small-study effects for sperm concentration (Fig. S5 in Appendix A).
3.2.3. Sperm progressive motility
Thirty-eight studies (4290 men) were included in the NMA; 34 were two-arm, two were three-arm, and two were four-arm trials. The network geometry is presented in Fig. S6 in Appendix A. Compared with a placebo or being untreated, CoQ10 (MD = 7.4%, 95%CI, 2.3%–12.4%), carnitine (MD = 7.5%, 95%CI, 2.1%–13.0%), SERM plus CoQ10 (MD = 11.0%, 95%CI, 0.1%–21.9%), and SERM plus androgen (MD = 9.0%, 95%CI, 1.2%–16.8%) resulted in significantly higher sperm progressive motility. When RCTs with a high risk of bias or without AT prior to sperm analysis were excluded, only CoQ10 still resulted in significantly higher sperm progressive motility under both conditions (Fig. S7 in Appendix A). The SUCRA values evaluated for each intervention for overall, excluded RCTs at high risk of bias, and excluded RCTs without AT are presented in Fig. S8 in Appendix A. The optimal intervention to improve sperm progressive motility was SERM plus kallikrein, even after trials without AT prior to sperm analysis had been excluded. The funnel plot indicated that there was a moderate small-study effect for sperm motility (Fig. S9 in Appendix A).
3.2.4. Sperm morphology
Twenty-four studies (2718 men) were included in the NMA; 20 were two-arm, two were three-arm, and two were four-arm trials. The network geometry is presented in Fig. S10 in Appendix A. Compared with a placebo or being untreated, pentoxifylline (MD = 8.5%, 95%CI, 3.8%–13.2%), CoQ10 (MD = 2.6%, 95%CI, 0.1%–5.1%), fatty acids (MD = 5.3%, 95%CI, 0.7%–9.9%), and SERM plus CoQ10 (MD = 11.0%, 95%CI, 4.6%–17.4%) resulted in significantly higher normal sperm morphology, while both pentoxifylline and fatty acids were still significant even after RCTs with a high risk of bias or without AT prior to sperm analysis (Fig. S11 in Appendix A). The SUCRA values evaluated for each intervention are presented in Fig. S12 in Appendix A. The optimal intervention for improving normal sperm morphology was SERM plus CoQ10, but shifted to pentoxifylline after excluding RCTs without AT prior to sperm analysis. The funnel plot indicated a lack of small-study effects for sperm normal morphology (Fig. S13 in Appendix A).
《3.3. Subgroup analysis》
3.3. Subgroup analysis
For specific sperm abnormality, only seven RCTs for oligospermia and six RCTs for asthenospermia were available, so we did not pool these data. Twenty-three RCTs for OA were available to perform the NMA. Compared with a placebo or being untreated, FSH, SERM, and carnitine plus CoQ10 significantly increased SPR (Fig. 3(d)). FSH, SERM plus CoQ10, and SERM plus kallikrein resulted in significantly higher sperm concentration (Fig. S3(d)). Androgen, CoQ10, carnitine, SERM plus vitamin C or E, and SERM plus CoQ10 resulted in significantly higher sperm progressive motility (Fig. S7(d)). Only SERM plus CoQ10 resulted in significantly higher normal sperm morphology (Fig. S11(d)). The optimal interventions for sperm concentration and progressive motility were SERM plus kallikrein and SERM plus CoQ10, respectively (Figs. S4(d) and S8(d)).
《3.4. Side effects》
3.4. Side effects
Thirty-six studies (55.4%) did not report on side effects, and 22 trials (33.8%) reported that there were no side effects or serious adverse events; the remaining seven trials (10.8%) were available for qualitative analysis. The commonly reported side effects included foul breath or bad taste (7.1%), nausea and vomiting (6.4%), diarrhea (6.4%), dyspepsia (5.6%), heartburn or reflux (5.3%), headache (4.0%), pruritis (2.6%), and dizziness and vertigo (1.6%). The risks of foul breath or bad taste (RR = 8.1, 95%CI, 1.0– 63.5) and vomiting (RR = 8.0, 95%CI, 1.0–63.0) were significantly increased when taking fatty acids and pentoxifylline, respectively (Table 2 [42,66,67,76,77]).
《Table 2》
Table 2 Adverse events.

《4. Discussion》
4. Discussion
Our study provides a comprehensive overview of the numerous treatments for idiopathic male infertility. It ranks the studies therapies in a single pooled analysis to identify the most effective therapy for idiopathic male infertility in terms of SPR and semen parameters. Studies on live birth are very limited, so no conclusion was available in this regard, and half of the trials did not report on side effects. However, our study indicated that carnitine plus vitamins is likely to be better than other therapies in achieving successful spontaneous pregnancy in couples in overall. FSH not only improved sperm quality, but also improved SPR, especially for men with OA. Although CoQ10 alone improved sperm motility, evidence for its effect on SPR is limited.
FSH and SERM have been empirically used for many decades for the treatment of male infertility, regardless of hormonal insufficiency [4]. Previously, a meta-analysis showed that FSH resulted in significantly higher SPR compared with a placebo, but it was not validated in assisted reproductive technique (ART) [10,90,91]. Another meta-analysis indicated that FSH significantly improved sperm concentration (MD = 3.17 × 106 mL–1 , 95%CI, 2.44 × 106 – 3.91 × 106 ) [10]; however, this change may have little clinical meaning, especially for severe oligozoospermia, which commonly results in lower SPR [92]. Here, we have demonstrated that FSH markedly increased sperm concentration and eventually resulted in a significantly higher SPR. The key roles of FSH in spermatogenesis include spermatogonial proliferation, metabolic and structural support, and the transport of nutritive substances to germ cells [93]. The effectiveness of FSH on sperm parameters depends on dosage and length of treatment [94]. A dose-dependent efficacy of FSH administration on sperm parameters has been demonstrated, where FSH only improved sperm motility at low doses (175.0–262.5 IU per week), while it increased concentration, total sperm count, and progressive motility at high doses (700– 1050 IU per week) [95]. On the other hand, it has been reported that sperm parameters significantly improved after more than four months of FSH administration [50]. Therefore, it is reasonable to consider that FSH has positive effects on both sperm quality and SPR, but its optimal dose and length of treatment are still unclear and warrant further verification in the future.
Despite not being approved by the FDA, SERM—mainly clomiphene and tamoxifen—has been widely used to treat idiopathic male infertility, both alone and in combination with other treatments, as they inhibit normal estrogenic negative feedback on the hypothalamus and pituitary gland and subsequently increase the secretion of FSH and luteinizing hormone (LH) [96]. Previous evidence has suggested that, compared with a placebo or being untreated, the use of clomiphene or tamoxifen not only resulted in significantly higher sperm concentration (MD = 5.24 × 106 mL–1 , 95%CI, 2.12 × 106 –88.37 × 106 ) and progressive motility (MD = 4.55%, 95%CI, 0.73%–8.37%), but also resulted in a higher SPR (odds ratio (OR) = 2.42, 95%CI, 1.47–3.94). Moreover, it was reported that 50 mg of clomiphene had a better effect than 25 mg [5]. In contrast, Cannarella et al. [97] showed that SERM had little effect on sperm concentration and motility, but improved sperm count and morphology and SPR compared with the controls (mixed with placebo, untreated, and/or other treatment). The discrepancy between these findings can probably be attributed to the controls. Interestingly, SERM in combination with CoQ10 significantly improved sperm quality, especially for men with OA. It has been demonstrated that CoQ10 plays an important role in both energy metabolism and lipid peroxidation in spermatozoa [98]. Supplementation of CoQ10 significantly increased not only the antioxidative capability of spermatozoa, but also sperm quality, such as motility; that is, there is a positive correlation between CoQ10 and sperm motility in infertile men [37,99]. Therefore, it seems plausible that SERM plus CoQ10 significantly improves sperm quality.
Nevertheless, our findings suggested that carnitine plus vitamins is the most effective regimen for achieving spontaneous pregnancy. Carnitine alone or combined with other treatments, such as vitamins, has been reported to improve both pregnancy and sperm quality. Carnitine is a water-soluble antioxidant mainly obtained from the diet; it plays a role in antioxidative stress and acts as an energy provider for sperm [100,101]. Thus, carnitine is one of the most used antioxidants for male infertility. At present, the vitamins used to treat male infertility mainly include vitamin C, vitamin E, and folic acid. However, direct evidence of only vitamins being used to treat male infertility is very limited, so the effectiveness of this treatment is largely unknown [102,103]. Although a meta-analysis showed a significant improvement in SPR due to the use of vitamin E in comparison with a placebo, this evidence was pooled from only one study that had a high risk of bias in its methodology, resulting in an overestimation of the efficacy [104]. However, vitamin C or E, acting as a common antioxidant, in combination with other agents (mainly zinc, selenium, carnitine, CoQ10, or SERM), is a very common treatment for male infertility, and some results show a significant improvement in sperm parameters, SPR, or both [105]. For example, carnitine plus vitamins significantly improved sperm motility compared with carnitine or vitamins individually [38,79]. However, any significant effect of vitamin C or E was not observed in our study as compared with a placebo, and carnitine plus vitamins being the most effective treatment to achieve successful spontaneous pregnancy may be due to other unknown mechanisms, such as sperm DNA fragmentation, decapacitation capacity, or seminal plasma contents. Further study is necessary to confirm the underlying mechanism.
Several limitations in the present study must be noted. First, data such as sperm parameters were not provided by all the studies investigated here; as a result, the data included in the analysis for sperm parameters were less than those for SPR. Second, due to the limited number of studies, we directly pooled data from patients with different types of sperm abnormality, dose or preparation of an agent, or length of treatment (e.g., FSH), rather than separating them into different categories, which may result in an imprecise effect estimate and rank for an intervention, especially for continuous data. Third, alternative medicines, such as herbal medicines and traditional Chinese medicines (TCMs), were not included in our current study. TCM generally consists of herbal medications and acupuncture, both of which may improve sperm parameters and pregnancy via regulating endocrine and antioxidant activity [106,107]. Difficulties in studying TCM include a generally low study quality and the contents of TCM still not being standardized or fully characterized. Finally, most interventions included in this NMA have few RCTs, resulting in limited network connectivity and statistical power. Although the ranking analysis provides a better overview of the treatments toward a specific outcome, it represents relative ranks rather than absolute differences between interventions; thus, the best treatment varies as a specific comparator [108].
In future, we recommend studies that compare SERM (preferably for 50 mg clomiphene) combined with carnitine, CoQ10, or other antioxidants under a rigorous methodology to validate the effects of SERM plus antioxidants on pregnancy outcomes. The dosage of antioxidants and the length of treatment should be simultaneously clarified, especially for L-carnitine, which has been reported to have toxicity at a high dosage; for example, 50 mg·mL–1 of L-carnitine was found to be toxic to sperm and significantly decreased sperm motility [109]. Furthermore, the endpoint of biochemical or clinical pregnancy may be insufficient; instead, it would be better to follow up the outcome measures to ongoing pregnancy and live birth [110]. Also, the report of outcomes should be described in detail according to the CONSORT guideline [111], especially regarding side effects. Although idiopathic male infertility is not a uniform disorder, the term was used here to maximize the number of included studies. In the subgroup analysis, the findings indicated that the optimal treatment for each outcome was largely different between specific sperm abnormality and overall; therefore, a specific sperm abnormality must be clearly defined within the diagnosis of idiopathic male infertility.
In conclusion, the optimal treatment for male infertility for live birth is still unknown. Carnitine plus vitamins and FSH are likely to be better than other therapies in achieving successful spontaneous pregnancy in couples with infertile men overall and with men with OA, respectively. The efficacy of other treatments on pregnancy outcomes warrants further verification, and the optimal dosage and length of treatment should simultaneously be identified.
《Acknowledgments》
Acknowledgments
The authors are grateful to Rui Wang (Department of Obstetrics and Gynecology, Monash Medical Centre, Australia) for expert advice on data analysis. The work was supported by the National Public Welfare Projects for Chinese Medicine (201507001) to Xiao Ke Wu; Theme-based Research Scheme (T13-602/21-N) from Research Grant Council and the Health and Medical Research Fund (06170246) from Food and Health Bureau to Chi Chiu Wang.
《Authors’ contributions》
Authors’ contributions
Jian Li, Xiao Ke Wu, Ernest Hung Yu Ng, and Chi Chiu Wang contributed to the study conception and design; Jian Li and Qi Wu collected the data; Jian Li and Chi Chiu Wang analyzed the data; Jian Li, Qi Wu, Ernest Hung Yu Ng, Xiao Ke Wu, and Chi Chiu Wang interpreted the work; and Jian Li drafted the manuscript.
Ernest Hung Yu Ng, Xiao Ke Wu, Ben Willem J. Mol, and Chi Chiu Wang critically revised the manuscript for important intellectual content; All authors commented on the drafts and approved the final draft; Jian Li and Chi Chiu Wang are the guarantors.
《Compliance with ethics guidelines》
Compliance with ethics guidelines
Jian Li, Qi Wu, Ernest Hung Yu Ng, Ben Willem J. Mol, Xiao Ke Wu, and Chi Chiu Wang declare that they have no conflict of interest or financial conflicts to disclose.
《Appendix A. Supplementary data》
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eng.2021.07.009.














 京公网安备 11010502051620号
京公网安备 11010502051620号




