《1. Introduction》
1. Introduction
The gut barrier plays a crucial role in maintaining host intestinal homeostasis by separating internal organs from harmful entities and signaling the immune cells to accommodate the microbiota, thereby perpetuating the normal function of the body [1]. Alterations in gut barrier integrity can lead to the development of gastrointestinal, autoimmune, and inflammatory diseases, as well as cancer caused by a leaky gut. Recent studies have shown that intestinal barrier dysfunction is also strongly correlated with metabolic diseases including obesity, diabetes, hypertension, and atherosclerosis [2-4]. Notably, increased gut barrier permeability results in an increased antigenic load entering the epithelial submucosa and leakage of bacterial metabolites or harmful molecules into the blood circulation. This consequently activates the inflammatory signaling pathways and stimulates the production of osteoclast cytokines, which aggravate osteoporosis [5]. Moreover, the integrity of the gut barrier function plays a significant role in alcoholic liver disease. A leaky intestinal barrier permits translocation of viable bacteria and microbial products to the liver, where it induces and promotes inflammation, as well as contributes to hepatocyte death and the fibrotic response [6].
Probiotics have been reported to have beneficial effects on the regulation of the immune and metabolic systems [7]. A number of animal studies have shown that oral administration of Bifidobacterium longum (B. longum) [8], Lactobacillus rhamnosus (L. rhamnosus) [9], and Lactobacillus reuteri (L. reuteri) [10], significantly increased bone mineral density (BMD) and bone mass. A mixture of three lactobacilli prevents the loss of cortical bone and increases bone resorption induced by ovariectomy [11]. Several evidences based on clinical trials suggested either single or multispecies probiotic supplement can alleviate osteoporosis [12,13]. A randomized double-blind placebo-controlled clinical study performed on 50 patients with osteopenia aged 50–72 years showed that a probiotic supplement containing bacteria from seven species can suppress bone resorption and bone turnover through reducing bone alkaline phosphatase (BALP) [14]. In addition, the supplementation with the supernatants of L. rhamnosus strain GG (LGG), VSL#3, and Akkermansia muciniphila (A. muciniphila) has been reported to reverse intestinal leakiness, systemic inflammation, and hepatic injury in a mouse model of alcohol-related liver disease [15,16]. A recent review also indicated that treatment with probiotics can alleviate hepatic inflammation by restoring gut barrier function, inhibiting oxidative stress, and decreasing endotoxin levels in the blood [17]. Meanwhile, clinical dates also indicated that treatment with probiotics could alleviate liver injury [18,19]. A cocktail of Bifidobacterium bifidum (B. bifidum) and Lactobacillus plantarum (L. plantarum) significantly altered the serum levels of alanine transferase (ALT), low-density lipoprotein, and total bilirubin in alcoholic liver disease patients [20]. However, the screening of the abovementioned functional bacterial strains has certain limitations. Most of the screening simply focused on famous commercial probiotics, such as LGG or VSL#3 [15,21]. Otherwise, the strains were screened out based on common biological parameters of probiotic such as the antioxidant activity, short-chain fatty acids (SCFAs) production, and gastrointestinal tolerance, which are not especially suitable for the screening of strains for protection against metabolic dysfunctions [22,23].
Probiotics play an important role in maintaining intestinal barrier function [24,25]. A number of studies based on animal models have demonstrated that supplementation with probiotics, such as Saccharomyces boulardii [26], L. reuteri [27], and Lactobacillus paracasei [28], can contribute to alleviating gut barrier injury caused by dextran sulfate sodium (DSS). Oral administration of B. longum showed significant protective effects against intestinal mucosal damage in western diet-treated mice [29]. In addition, several clinical trials indicated that oral supplementation with specific probiotics can restore gut barrier dysfunction in patients with obesity [30], irritable bowel syndrome [31], and necrotizing enterocolitis [32]. We noticed that several probiotics exert beneficial effect by interacting directly with the gut barrier. Numerous microbialassociated molecular patterns, include flagellin, pili protein, and peptidoglycan, regulate gut barrier function by activating Toll-like receptors and nucleotide-binding and oligomerization domainlike receptor pathways [3]. Moreover, a number of studies showed supplement with special probiotics can alleviate a variety of diseases, including ulcerative colitis, nonalcoholic fatty liver, and obesity, by modulating gut microbiota [33,34]. It is also worth mentioning that probiotics with excellent gut barrier regulation ability have also been reported to exhibit protection against metabolic diseases. Oral administration of heat-killed and live L. reuteri markedly reduced gut permeability, serum glucose, insulin, and lipid profiles in the serum and liver, thereby relieving obesity and diabetes [35]. Intake of a cocktail of L. plantarum 299v and B. lactis Bi07, which have been proven to significantly restore gut barrier function in vitro, can reduce infarct size, improve left ventricular function and alleviate cardiovascular disease in rats [36].
Differentiated Caco-2 cells, which possess a microvilli structure, tight junctions, and cell polarity similar to human small intestines, could spontaneously form a monolayer on the transwell membrane [37]. The in vitro monolayer model of Caco-2 cells dependent on the transwell membrane was recognized as a common in vitro method for the assessment of gut barrier function. A number of studies have used this model to evaluate the effects of various nutrients and materials, such as plant extracts [38], dairy products [39], and chitosan nanoparticles [40], on intestinal barrier function. Anderson et al. [41] compared the different protective effects of eight commercially used probiotics on gut barrier function based on the Caco-2 transwell appraisal model. A previous study using lipopolysaccharide (LPS)-induced Caco-2 cells indicated that A. muciniphila supplementation could recover the trans-epithelial electrical resistance (TEER) of the gut barrier and reverse gut barrier injury [42]. However, to the best of our knowledge, this model is mainly used for the assessment of probiotics for their gut barrier regulation abilities, with a very rare application for the further screening of strains against specific metabolic diseases.
Therefore, the aim of this study was to evaluate the applicability of the Caco-2 cell monolayer model for the screening of probiotics against metabolic diseases. Two types of metabolic diseases (osteoporosis and alcoholic liver disease) that are closely associated with intestinal barrier dysfunction were selected to determine the correlation between the regulation of probiotics of different species/ strains on gut barrier function in vivo and their protection against metabolic diseases. Based on these analyses, we sought to establish an efficient model for the screening of probiotics against specific metabolic diseases.
《2. Materials and methods》
2. Materials and methods
《2.1. Cell line and bacteria》
2.1. Cell line and bacteria
Caco-2 cells were purchased from the Institute of Biochemistry and Cell Biology (China). The culture and subculture were performed as described in a previous study [42]. All the tested 139 Lactobacillus strains belonging to eight species, including L. fermentum, L. casei, L. gasseri, L. reuteri, L. helveticus, L. rhamnosus, L. plantarum, and L. bulgaricus, were provided by the in-house Culture Collection of Food Microbiology, Jiangnan University (Wuxi, China). Some of the cultured strains were centrifuged and suspended in the cell culture medium for cell trials, and the other part was cultured and lyophilized for animal experiments as previously reported [43].
《2.2. In vitro experiments》
2.2. In vitro experiments
Three independent experiments were performed using in vitro assays. For the first TEER assay, Caco-2 cells were seeded into 12 mm cell culture inserts (0.4 μm pore size; Costar, USA) at a density of 1 × 105 . Monolayers of the cells were cultured for 18– 21 days until the value of TEER had stabilized. The cells were pretreated with tumor necrosis factor (TNF)-α (2 ng·mL–1 ) for 4 h, further washed, and supplemented with Lactobacillus strains (1 × 108 colony forming units (CFU) per well) for 4 h. For the second TEER assay, the culture process is described above, while differentiated Caco-2 monolayers were treated with 5% alcohol and Lactobacillus strains (1 × 108 CFU per well) for 24 h. The doses and handling times were selected based on previous studies [44,45]. For the detection of messager RNA (mRNA) expression of tight junction proteins (ZO-1, occludin, and claudin-1), confluent cells (grown in six-well plates) were treated as described in the second TEER assay, and the cells were harvested and subjected to real-time (RT) quantitative polymerase chain reaction (PCR) analysis. The procedure and primers were selected as previously reported [46].
《2.3. Animal》
2.3. Animal
Female Sprague Dawley rats (12 weeks of age) and male C57BL/6 mice (8 weeks of age) were purchased from the Shanghai Laboratory Animal Center (China). The feeding conditions were controlled at 23–25 °C and 40%–60% humidity under a 12 h light–dark cycle. All animals were provided with clean water and food during the adaptation phase. The protocol for the animal trials in this study was approved by the Ethics Committee of Jiangnan University, China (JN. No20190315S0750611 and JN. No20190430c0720714).
《2.4. Ovariectomy-induced osteoporosis trial》
2.4. Ovariectomy-induced osteoporosis trial
Fifty rats were randomly assigned to ten groups (control, mode, alendronate sodium, L. fermentum strains (5-1L, L9-10-3-1, and FZJTZ20M3), L. casei strains (F-FJ-ND-D12-L-9 and HN13-1), L. rhamnosus strain (LGG), and L. plantarum (CCFM8610)), with five rats in each group. The treatments for each group are indicated below.
Control: The rats underwent a sham operation and were gavaged with sterile saline a month later.
Mode: The rats underwent ovariectomy and were gavaged with sterile saline a month later.
Alendronate sodium: The rats underwent ovariectomy and were gavaged with a sterile solution of alendronate sodium at a dose of 1 mg·kg–1 a month later.
The bacterial treatment groups, namely 5-1L, L9-10-3-1, FZJTZ20M3, F-FJ-ND-D12-L-9, HN13-1, LGG, and CCFM8610, underwent ovariectomy and were gavaged with a sterile saline suspension of Lactobacillus strains (1 × 109 CFU·mL–1 per mouse per day) a month later.
The volume of gavage was 1.5 mL and was administered for four weeks. Ovariectomy means that the bilateral ovaries were exteriorized, ligated, and excised. Rats that underwent the sham operation had only a piece of fat excised. The doses of alendronate sodium and probiotics were selected based on previous studies [47,48]. After the whole experiment, all surviving rats were sacrificed using isoflurane overdose, and their femurs, uteri, blood, colons, and feces were collected. Uterus coefficient (%) = (mean uterus weight/mean body weight) × 100. BMD of femurs and other bone parameters, including cortical volume (Ct.V), bone volume fraction (BV/TV), and trabecular thickness (Tb.Th), were analyzed using micro-computed tomography (micro-CT), and other femurs were fixed in 4% paraformaldehyde to perform a histological evaluation as previously described [48]. Gas chromatography-mass spectrometry (GCMS-QP2010 Ultra system; Shimadzu Corporation, Japan) was used to analyze SCFAs concentrations, as previously described [49]. Total RNA was collected and extracted through Total RNA Isolation Kit (Vazyme Biotech Co., Ltd., China). RNA reverse transcription was performed by the qPCR Reagent Kit (Vazyme Biotech Co., Ltd.). ZO-2, occludin, and claudin-1 mRNA expression in the colon was detected using an RT-PCR system (CFX Connect; Bio-Rad, USA) as previously described [50]. The levels of estradiol (E2), endotoxin (ET), tartrate resistant acid phosphatase (TRACP), BALP, colon tumor cell line (CTX-I), transforming growth factor (TGF)-β1, TNF-α, and Interleukin (IL)-10 were measured using an enzyme-linked immunosorbent assay (ELISA) kit (R&D, UK).
《2.5. Alcohol-induced liver injury trial》
2.5. Alcohol-induced liver injury trial
Seventy mice were randomly assigned to seven groups (control, alcohol, L. rhamnosus strain (LGG), L. casei strains (RS8-5 and VCQRC7-161-M2), L. helveticus strains (D-7-S146 and 8M-10)), with ten mice in each group. The treatments for each group are given below.
Control: The mice were administered a control liquid for nine weeks and were gavaged with sterile saline five weeks later.
Alcohol: The mice were administered the mode liquid for nine weeks and were gavaged with sterile saline five weeks later.
Bacteria treatment group: The mice of the LGG, RS8-5, VCQRC7-161-M2, D-7-S146, and 8M-10 groups were administered the mode liquid for nine weeks and were gavaged with a sterile saline suspension of Lactobacillus strains (1 × 109 CFU·200 μL–1 per mouse per day) five weeks later.
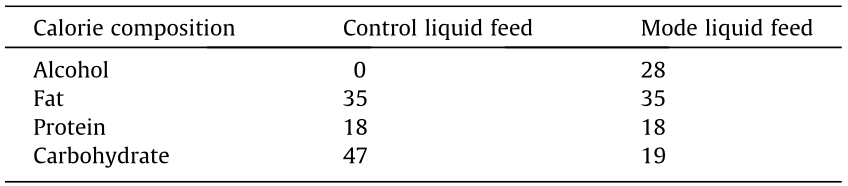
The volume of gavage was 0.2 mL and the feed formula is shown in Table 1. After the whole experiment was completed, the mice were sacrificed using isoflurane overdose, and their livers, blood, and ilea were collected. The liver index was calculated using the following formula: liver index (g·100 g–1 body weight) = mean liver weight (g)/mean body weight (100 g). The levels of IL-6, IL-1β, and endotoxin in the livers were detected using ELISA kits (R&D). The mRNA expression of tight junction proteins in the ileum was determined using an RT-PCR system (CFX Connect; Bio-Rad), as previously described [51]. The levels of glutathione (GSH), superoxide dismutase (SOD), and malondialdehyde (MDA) in the liver were measured using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, China). The activities of aspartate transferase (AST), ALT, gamma-glutamyl transpeptidase ( -GT), total cholesterol (TC), and triglyceride (TG) in the serum were detected using an automatic biochemical analyzer (TBA-40FR, Toshiba Medical, Japan). The livers were fixed in 4% paraformaldehyde for histological evaluation, as previously described [52].
-GT), total cholesterol (TC), and triglyceride (TG) in the serum were detected using an automatic biochemical analyzer (TBA-40FR, Toshiba Medical, Japan). The livers were fixed in 4% paraformaldehyde for histological evaluation, as previously described [52].
《Table 1》
Table 1 Liquid feed formula of alcohol-induced liver injury trial (%).
《2.6. Statistical analysis》
2.6. Statistical analysis
The data are expressed as the mean ± standard deviation (SD). One-way analysis of variance was used to analyze the results, followed by Tukey’s post-hoc test. Statistical significance was set at P < 0.05. Statistical analyses of the data obtained were performed using SPSS software (version 16.0; SPSS Inc., USA). Correlations between variables were assessed using Pearson’s correlation coefficient. A strong correlation was defined as a correlation coefficient of r ≥ 0.8.
《3. Results》
3. Results
《3.1. The effects of Lactobacillus strains on the barrier function in TNFa-treated Caco-2 cell monolayer》
3.1. The effects of Lactobacillus strains on the barrier function in TNFa-treated Caco-2 cell monolayer
Compared with the control treatment, TNF-α exposure induced a marked reduction in TEER in the Caco-2 cell monolayer (Fig. 1). Treatment with different Lactobacillus strains exhibited varied effects on the regulation of barrier function. The degree of recovery of the TEER of strains with superior regulation ability, such as L. fermentum 5-1L, L. casei F-FJ-ND-D12-L-9, L. plantarum CCFM8610 and LGG, were close to 90%, while that of other strains ranged from –50% to 80% (the degree of recovery of the TEER is obtained through comparing treated group with TNF-α group).
《Fig. 1》
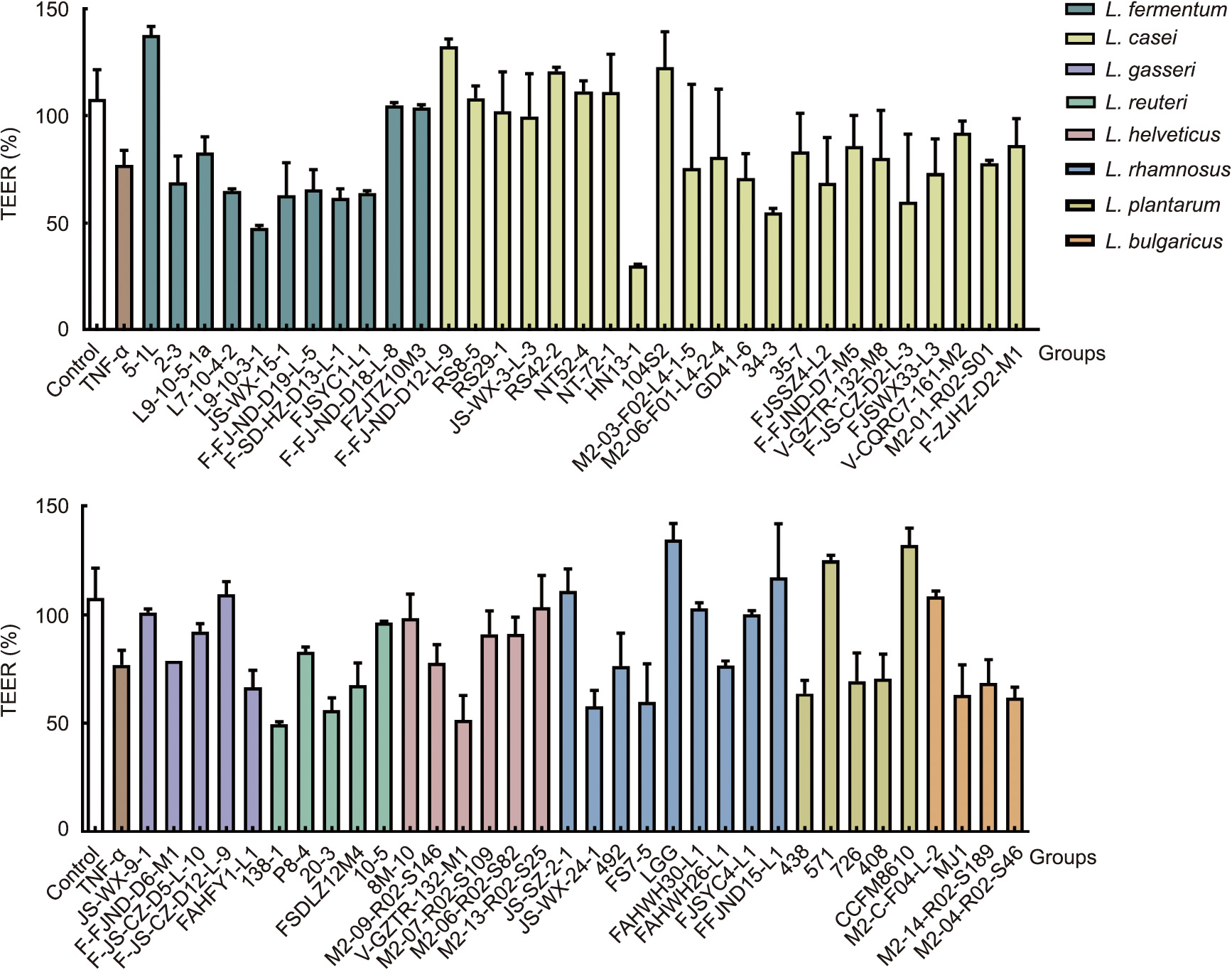
Fig. 1. The effect of Lactobacillus strains on TEER in Caco-2 cell monolayer membranes after treatment with TNF-α (2 ng·mL–1 ).
《3.2. The effects of Lactobacillus strains with different regulative abilities on gut barrier function against ovariectomy-induced osteoporosis in rats》
3.2. The effects of Lactobacillus strains with different regulative abilities on gut barrier function against ovariectomy-induced osteoporosis in rats
Three L. fermentum strains possessing different regulatory abilities on TEER were selected for further evaluation of their protective effects against osteoporosis (Fig. 2(a)). Oral supplementation of L. fermentum 5-1L provided remarkable beneficial effects on the indicators of weight and bone structure including BMD, BV/TV, Ct.V, and Tb.Th (P < 0.05), while the treatment with other L. fermentum strains (L9-10-3-1 and FZJTZ20M3) did not show the same significant effect (Figs. 2(b)–(d) and Figs. S1(a) and (b) in Appnedix A). L. fermentum 5-1L was also more effective in reducing the levels of TRACP, BALP, CTX-I, and ET, and increased the expression of ZO-1, Occludin, and Claudin-1 (Figs. 2(e)–(h) and Fig. S1(d) in Appendix A) compared with the other tested strains. As shown in Fig. 2(i), ovariectomy induced the reduction of the bone cells and enlargement of bone trabecula. Treatment with L. fermentum 5-1L had a more significant effect on the recovery of these alterations than L9-10-3-1 and FZJTZ20M3. Similar to the strain-specific effects of L. fermentum, the results of different L. casei strain treatments also showed that the in vivo protection against ovariectomy-induced osteoporosis was related to the regulatory abilities of probiotics on TEER in Caco-2 cell monolayers (Fig. S2 in Appendix A).
《Fig. 2》
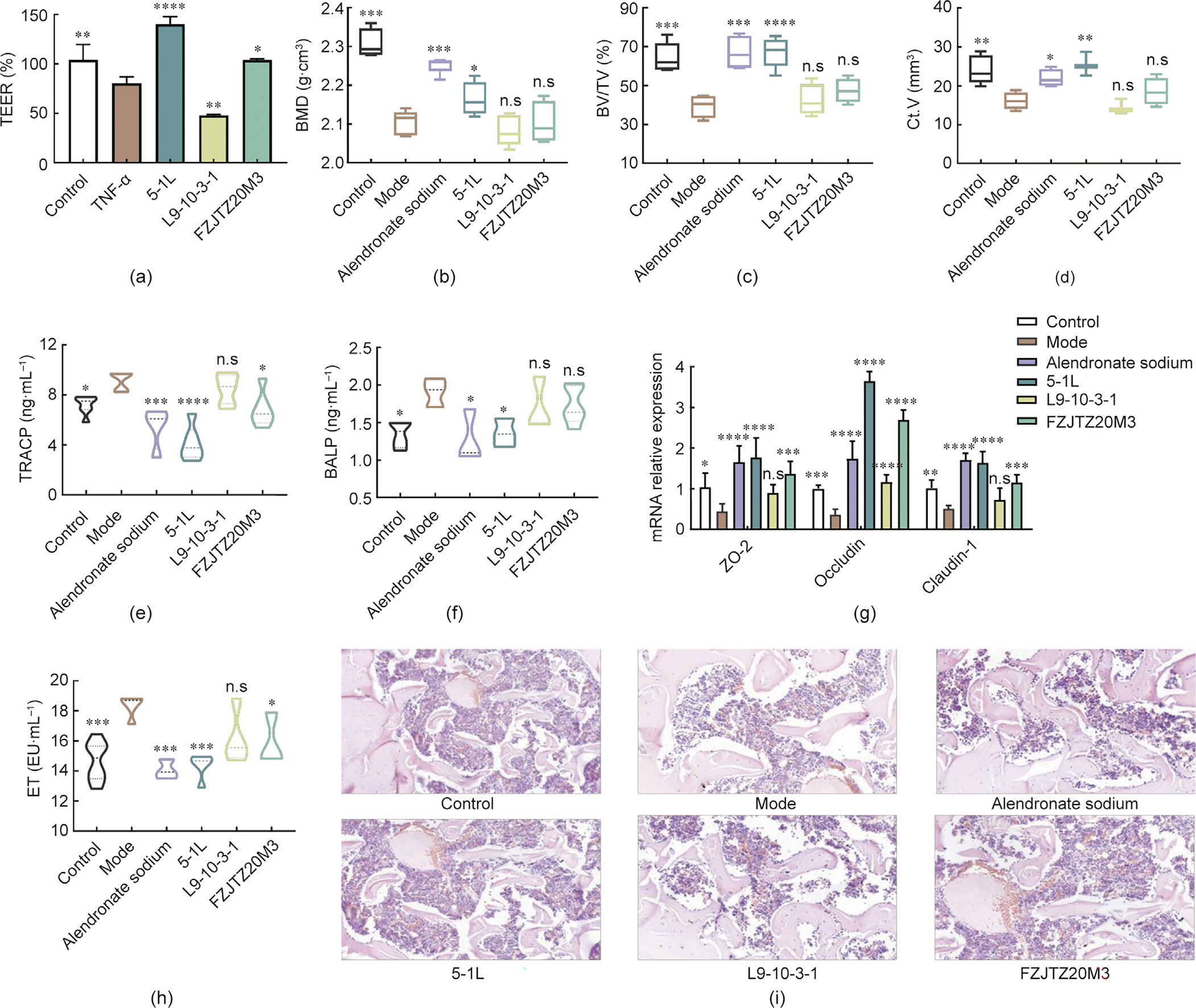
Fig. 2. The effect of L. fermentum strains on alleviating osteoporosis in rats. (a) TEER in Caco-2 cell monolayer membranes. (b) BMD of femurs. (c) BV/TV of femurs. (d) Ct.V of femurs. (e) TRACP levels in serum. (f) BALP levels in serum. (g) mRNA expression levels of tight junction protein in colon. (h) ET levels in serum. (i) Histological examination of representative image of hematoxylin and eosin (H&E) staining in femurs of rats. Mode: received ovariectomy. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, and n.s., not significant versus ovariectomy groups. EU: ET unit.
《3.3. Analysis of the correlation between the ability to regulate intestinal barrier in vitro and osteoporosis-alleviating effects in vivo of Lactobacillus strains》
3.3. Analysis of the correlation between the ability to regulate intestinal barrier in vitro and osteoporosis-alleviating effects in vivo of Lactobacillus strains
As shown in Fig. 3, the results of Pearson correlation analysis indicated that the concentrations of TRACP (rXY = –0.93) and CTX-I (rXY = –0.81) were significantly negatively correlated with the value of TEER, while the parameters in vitro, such as BV/TV (rXY = 0.85) and claudin-1 (rXY = 0.87), were significantly positively correlated with the TEER value. Meanwhile, the results of principal component analysis revealed that the indicators between in vivo and in vitro have a strong correlation and rXY = 0.82.
《Fig. 3》
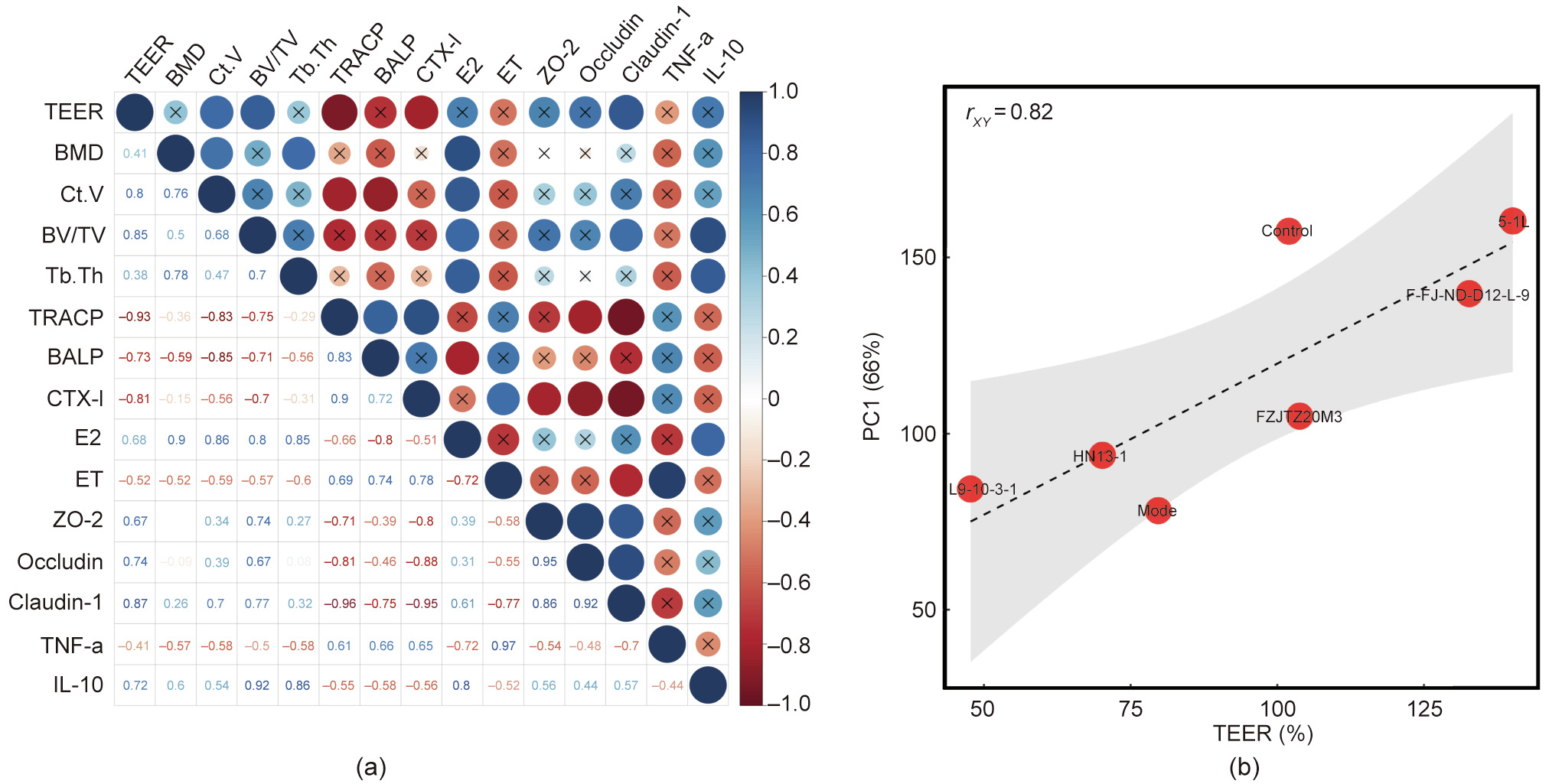
Fig. 3. (a) Correlation between the TEER value and each tested parameters in osteoporosis trial. (b) Correlation between the TEER value and PC1. PC1: the first principal component was obtained by principal component analysis of all tested parameters in osteoporosis trial.
《3.4. The verification of the correlation between the gut barrier regulation ability and the protection against osteoporosis of probiotics》
3.4. The verification of the correlation between the gut barrier regulation ability and the protection against osteoporosis of probiotics
Based on the results of Fig. 2, Figs. S1 and S2, we found out that the effects of L. fermentum and L. casei against osteoporosis were positively correlated with their intestinal barrier regulation in vitro. To further illustrated whether this correlation also exists in other species, we selected another two species of Lactobacillus (L. rhamnosus LGG and L. plantarum CCFM8610) to verify the correlation between the gut barrier regulation ability and the protection against osteoporosis of probiotics (Fig. 4 and Fig. S3 in Appendix A). Considering all the tested indicators shown in Fig. 4, both LGG and CCFM8610 provided significant protective effects against the symptoms of osteoporosis (P < 0.05).
《Fig. 4》

Fig. 4. The effect of Lactobacillus strains (LGG and CCFM8610) on alleviating osteoporosis in rats. (a) TEER in Caco-2 cell monolayer membranes. (b) BMD of femurs. (c) BV/TV of femurs. (d) Tb.Th levels in serum. (e) ET levels in serum. (f) E2 levels in serum. (g) mRNA expression levels of tight junction proteins in the colon. (h) BALP levels in serum. Mode: received ovariectomy. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, and n.s., not significant versus ovariectomy groups.
《3.5. The effects of Lactobacillus strains on the barrier function in alcohol-treated Caco-2 cell monolayers》
3.5. The effects of Lactobacillus strains on the barrier function in alcohol-treated Caco-2 cell monolayers
As shown in Fig. 5, alcohol treatment significantly decreased the TEER value in Caco-2 cell monolayers when compared to the control treatment. The degree of recovery was between 10%–110% after the addition of the tested probiotic strains.
《Fig. 5》
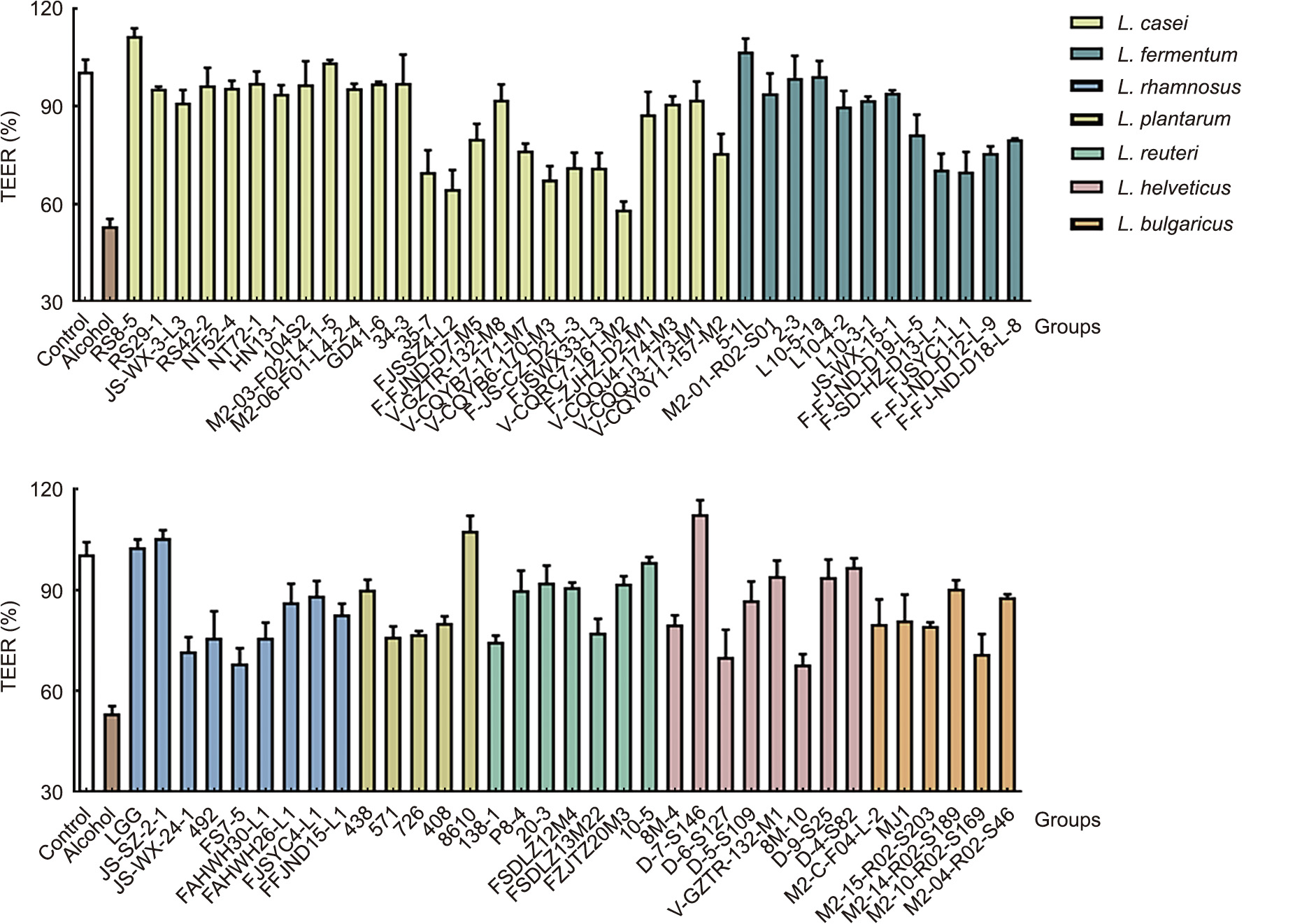
Fig. 5. The effect of Lactobacillus strains on TEER in Caco-2 cell monolayer membranes after treatment with 5% alcohol.
《3.6. The effects of Lactobacillus strains with different regulative abilities on gut barrier function against chronic alcoholic liver disease in mice》
3.6. The effects of Lactobacillus strains with different regulative abilities on gut barrier function against chronic alcoholic liver disease in mice
The L. casei RS8-5 strain with a superior ability to regulate gut barrier function (110% recovery rate on TEER value and significant effects on tight junction protein expression), and another strain (VCQRC7-161-M2) with non-significant protective effects on the gut barrier (Figs. 6(a) and (b)) were selected to evaluate their alleviative effects against alcoholic liver disease. Treatment with L. casei V-CQRC7-161-M2 exhibited no beneficial effects on liver function and serum lipid parameters, namely ALT, AST,  -GT, TG, and TC, whereas oral administration of RS8-5 and LGG markedly affected the recovery of these indicators (Figs. 6(c)–(e) and Fig. S4 in Appendix A). Compared with L. casei V-CQRC7-161-M2, RS8-5 significantly reduced the oxidative stress by increasing SOD (P < 0.01) and GSH (P < 0.001) expression and decreasing MDA production (P < 0.001) (Figs. 6(f) and (g) and Fig. S4). Treatment with RS8-5 also showed a more effective regulation on the levels of serum endotoxin and TNF-α than treatment with V-CQRC7-161-M2 (Figs. 6(h) and (i)). It was noted that both LGG and RS8-5 could significantly alleviate alcohol-induced liver pathological injury, while V-CQRC7-161-M2 failed to provide similar protection (Fig. 6(j)). Moreover, supplement with RS8-5 can markedly decrease liver index in mice exposed to chronic alcohol (Table S1 in Appendix A). Different L. helveticus strain treatments also showed that the in vivo protective effects against alcohol-induced chronic alcoholic liver disease were related to the regulatory abilities of probiotics on TEER in Caco-2 cell monolayers (Fig. S5 in Appendix A).
-GT, TG, and TC, whereas oral administration of RS8-5 and LGG markedly affected the recovery of these indicators (Figs. 6(c)–(e) and Fig. S4 in Appendix A). Compared with L. casei V-CQRC7-161-M2, RS8-5 significantly reduced the oxidative stress by increasing SOD (P < 0.01) and GSH (P < 0.001) expression and decreasing MDA production (P < 0.001) (Figs. 6(f) and (g) and Fig. S4). Treatment with RS8-5 also showed a more effective regulation on the levels of serum endotoxin and TNF-α than treatment with V-CQRC7-161-M2 (Figs. 6(h) and (i)). It was noted that both LGG and RS8-5 could significantly alleviate alcohol-induced liver pathological injury, while V-CQRC7-161-M2 failed to provide similar protection (Fig. 6(j)). Moreover, supplement with RS8-5 can markedly decrease liver index in mice exposed to chronic alcohol (Table S1 in Appendix A). Different L. helveticus strain treatments also showed that the in vivo protective effects against alcohol-induced chronic alcoholic liver disease were related to the regulatory abilities of probiotics on TEER in Caco-2 cell monolayers (Fig. S5 in Appendix A).
《Fig. 6》
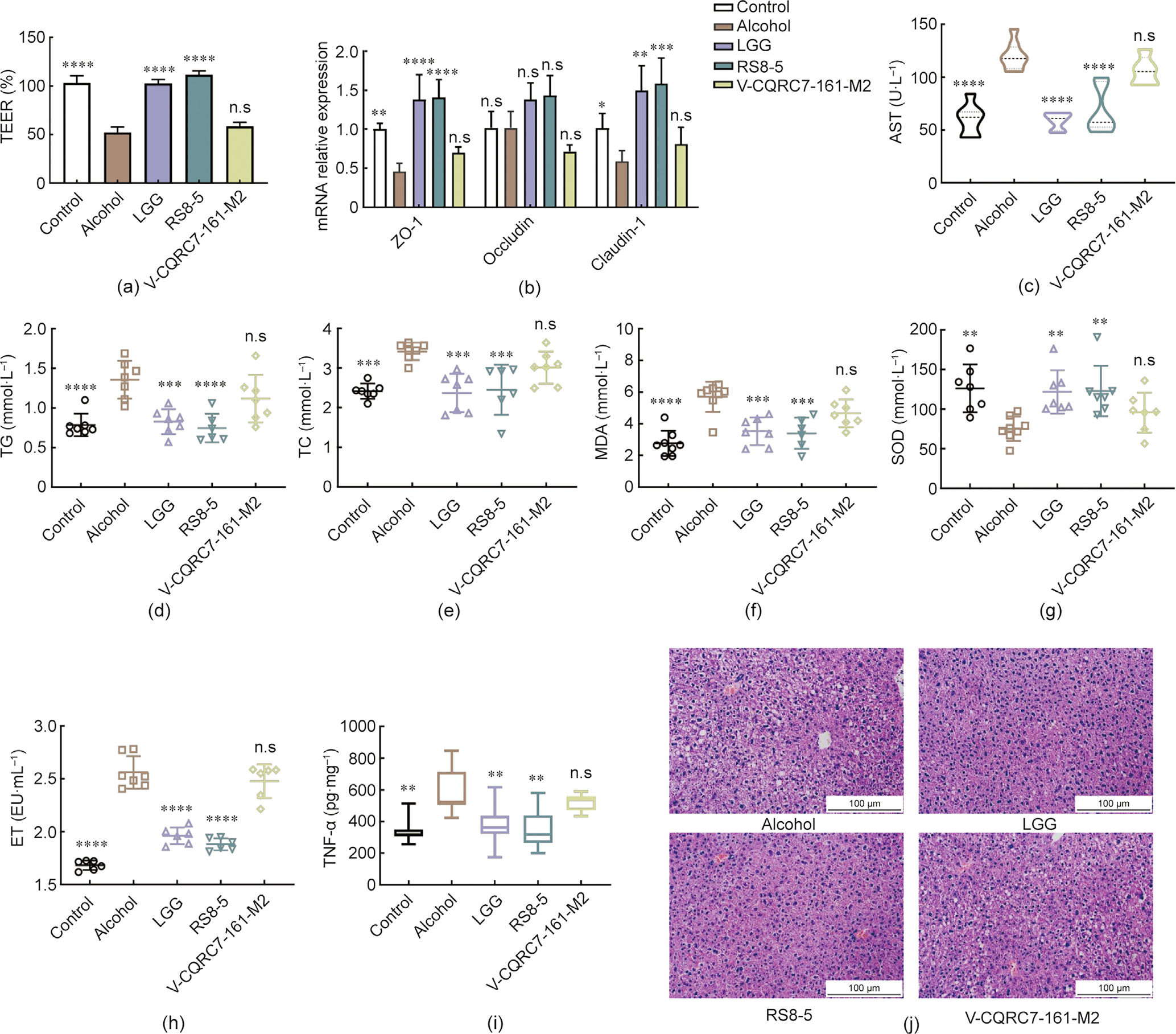
Fig. 6. The effect of L. casei strains on alleviating alcoholic liver disease in mice. (a) TEER in Caco-2 cell monolayer membranes. (b) mRNA expression levels of tight junction proteins in Caco-2 cells. (c) AST levels in serum. (d) TG levels in serum. (e) TC levels in serum. (f) MDA levels in serum. (g) SOD levels in serum. (h) ET levels in serum. (i) TNF-α levels in the livers. (j) Histological examination of representative image of H&E staining in the livers of mice. **P < 0.01; ***P < 0.001; ****P < 0.0001, and n.s., not significant versus ovariectomy groups.
《3.7. Analysis of the correlation between the ability to regulate intestinal barrier in vitro and alcoholic liver injury-alleviating effects in vivo of Lactobacillus strains 》
3.7. Analysis of the correlation between the ability to regulate intestinal barrier in vitro and alcoholic liver injury-alleviating effects in vivo of Lactobacillus strains
The results of the Pearson correlation analysis indicated that the indicators in vitro, including tight junction proteins, SOD, and GSH, were significantly positively correlated with the value of TEER in vivo (rXY > 0.9). Other indices related to the severity of alcoholic liver disease, namely endotoxin, ALT, AST, TC, and MDA were significantly negatively correlated with the value of TEER (rXY < –0.9) (Fig. 7(a)). In addition, the results of principal component analysis revealed that the indicators between in vivo and in vitro have strong correlation and the rXY = –0.97 (Fig. 7(b)).
《Fig. 7》
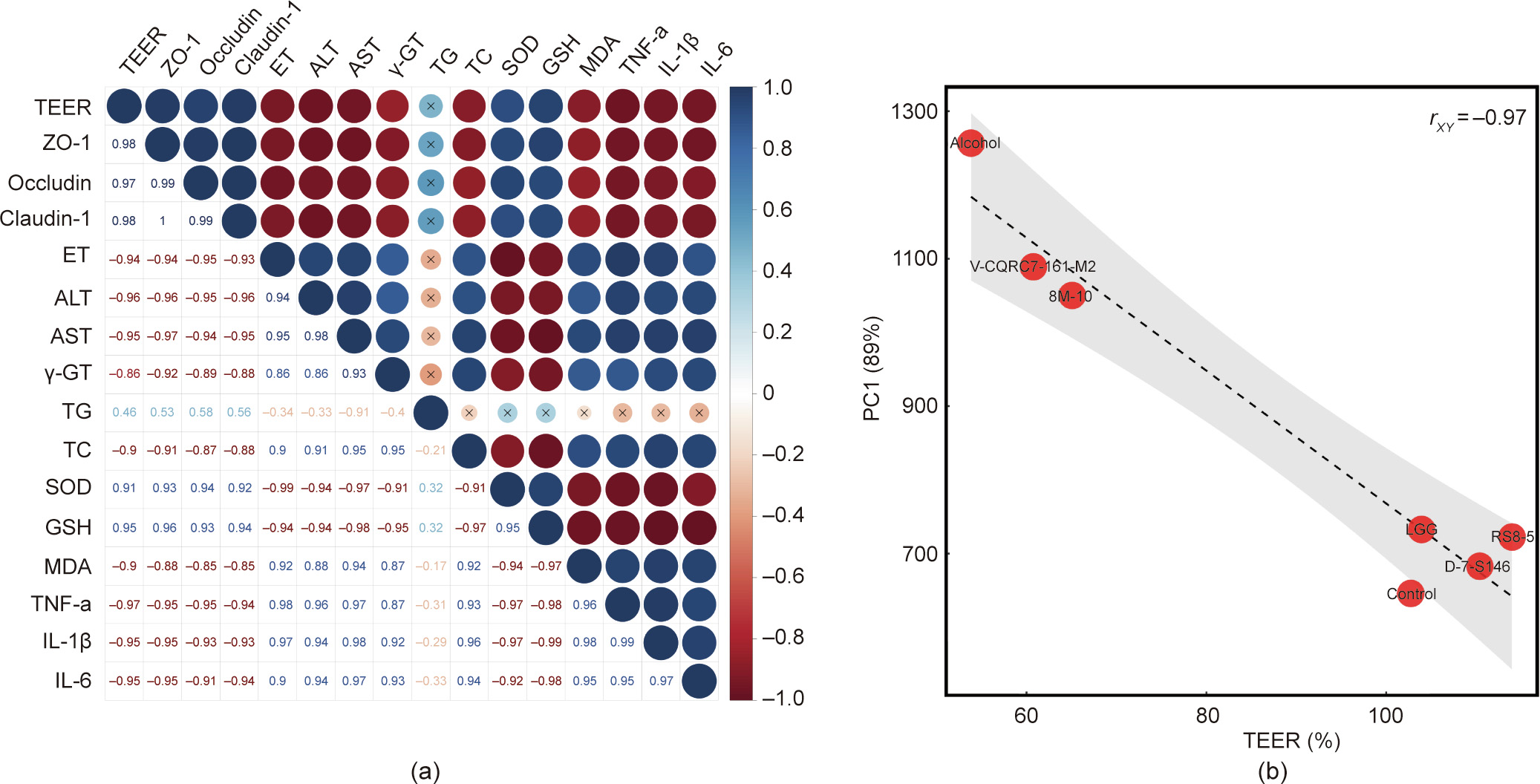
Fig. 7. (a) Correlation between the TEER value and each tested parameters in alcoholic liver injury trial. (b) Correlation between the TEER value and PC1.
《4. Discussion》
4. Discussion
《4.1. The selection of methods for intestinal barrier dysfunction》
4.1. The selection of methods for intestinal barrier dysfunction
In this study, the regulatory effects of Lactobacillus strains on intestinal barrier function were investigated by determining the TEER of Caco-2 cell monolayers. We further evaluated the correlation between the regulative abilities on gut barrier function and the protection against specific metabolic diseases of probiotics. Caco-2 cells are human epithelial colorectal adenocarcinoma cells with a structure and function similar to the differentiated small intestinal epithelial cells and can spontaneously form a monolayer on the transwell membrane [37]. Therefore, cultured Caco-2 cell monolayers were utilized as an in vitro model for testing the gut barrier function. TEER measurement is a commonly used electrophysiological approach to quantify tight junction permeability based on the impedance of cell monolayers. Intestinal epithelial cells have been reported for TEER measurement to reflect gut barrier function; the advantages of this method are that it is noninvasive and can be used to monitor live cells in real-time [53]. TNF-α has been reported to play an important role in the pathogenesis of osteoporosis. The increase of TNF-a level was observed in patients with osteoporosis and another study showed that bone loss did not occur in mice lacking TNF-α receptor [54,55]. The underlying mechanism may be that TNF-α synergistically promotes RANKL (nuclear factor (NF)-κB ligand)-induced osteoclast formation through activation of NF-κB and PI3K/Akt signaling [56]. TNF-α has also been reported to significantly reduce the TEER of the Caco-2 monolayer in a dose-dependent manner at 2–10 ng·mL–1 , indicating that it is an important factor for the increase in intestinal permeability [44]. Ovariectomy, which could induce a decrease in estrogen levels in vivo, is recognized as a common method to induce osteoporosis in animal [14]. The deficiency of estrogen can induce T-cell to produce TNF-α, which enhanced macrophage colony-stimulating factor- and RANKL-induced osteoclastogenesis through activating the TNF-α receptor p55 [57]. However, ovariectomy is only limited in vivo, we selected TNF-a to simulate osteoporosis in cell models. Since the main purpose of the study is to establish an in vitro model to efficiently screen potential probiotics against specific metabolic diseases, we only considered the effect of bacteria on the barrier but did not investigate the action mode of probiotic on regulating barrier in detail. Alendronate sodium, a commonly used drug in the clinical treatment to relieve osteoporosis through suppressing bone resorption by inducing osteoclast inactivation, was selected in the present animal study as a positive control [58]. For the simulation of alcoholic liver disease in vitro, we noticed that high-dose treatment with alcohol causes impairment of the gut barrier and increases intestinal permeability, further disrupting the balance in gut microbiota and induced endotoxemia, finally leading to alcoholic liver damage [59]. Previous studies have shown that alcohol exposure could break the tubulin of Caco-2 cells at a concentration of only 2.5%, and there was a dose-dependent relationship between alcohol concentration and the increase in monolayer membrane permeability of Caco-2 cells [60,61]. For animal trials, the Lieber–DeCarli diet is commonly used to mimic chronic alcohol abuse [62]. Considerable evidence indicates that treatment with LGG regulates the mucosal immune system, enhances the intestinal barrier function, inhibits endotoxin production, and alleviates alcoholic liver injury [44,63,64]. Therefore, this probiotic strain was used as a positive control in the present study.
《4.2. Strain-dependent regulative activity against intestinal barrier dysfunction in vitro 》
4.2. Strain-dependent regulative activity against intestinal barrier dysfunction in vitro
Based on our data from the in vitro trial, different Lactobacillus strains exhibited a significant strain specificity for the intestinal barrier regulation abilities but not species-specificity (Figs. 1 and 5). Consistent with our results, treatment with L. rhamnosus HN001 exhibited a more significant effect on TEER values than the seven others commercially used probiotic strains in a Caco-2 cell monolayer model [41]. An animal study showed that treatment with Bacteroides fragilis (B. fragilis) FSHCM14E1, a strain possessing a better SCFA secretion ability than other B. fragilis strains, provided the most superior effects against intestinal barrier injury induced by DSS [65]. The specific molecules produced by probiotics, such as organic acids, extracellular vesicles, and secreted proteins, may differentially interact with signaling pathways that play important roles in enhancing gut barrier function (such as NF-κB, myosin light chain kinase, mitogen-activated protein kinase, and protein kinase A pathway) [66]. This may partly explain the strain-specific regulatory effect of different probiotics on the barrier function of the Caco-2 cell monolayer. Different Lactobacillus existed a significant discrepancy on physiological properties, including genetic constitution, glucose utilization, and environmental resistance [67]. Previous studies have indicated that probiotics belonging to the same species, such as Bifidobacterium [68], Bacteroides [65], and Akkermansia [69], showed discrepant regulatory effects on intestinal health. However, several studies showed the protective effect of probiotics against constipation or non-alcoholic fatty liver disease existed interspecific difference [70,71]. The function of probiotics may be related to complicated factors, such as the biological properties of the bacteria, the interactions of the strains and the host, and the action mode of the probiotics in vivo [72]. These factors may lead to the controversial results of the interspecific effects of probiotics on the host. Therefore, to avoid the interference of different species and to more integrally evaluate the ability of Lactobacillus on intestinal barrier function regulation, we selected three species (L. fermentum, L. casei, and L. helveticus) of Lactobacillus to respectively conduct animal trials. In the study, a relatively larger number of tested strains were selected for the mentioned three species than other species of probiotics. Meanwhile, the regulative ability on intestinal barrier existed huge difference in these three species. In addition, several previous studies based on animal trials indicated that treatment with these three species could significantly reverse intestinal barrier dysfunction [73,74].
《4.3. Strain-dependent regulative activity against intestinal barrier dysfunction in vivo 》
4.3. Strain-dependent regulative activity against intestinal barrier dysfunction in vivo
The indicators of bone structure including BMD, Ct.V, and BV/TV as well as the indicators of tight junction protein, which can be regarded as parameters positively related to the alleviation of osteoporosis, have a higher weight than other indicators in the overall evaluation. Therefore, the composite indicator of these parameters through principal component analysis (PCA) was positive. PCA is a method to make the original multiple indicators into a new comprehensive index through linear combination [75]. Since the correlation coefficients is obtained by analyzing the degree of correlation between TEER and composite indicator of animal trial, the correlation coefficients were 0.82 in osteoporosis and alcoholic liver injury (Fig. 2, and Figs. S1 and S2). An earlier report provided direct in vivo evidence that L. fermentum was able to prevent stress-induced intestinal barrier dysfunction and exert beneficial effects on the regulation of the systemic immune response [76]. L. fermentum-fermented milk products were reported to alter the gene expression levels of bone metabolism-related markers and downregulate boneapoptosis-related genes stimulated by ovariectomy [77]. Moreover, treatment with L. casei had a preventative effect on bone loss by decreasing the activity of tartrate-resistant acid phosphatase in ovariectomized rats [78]. The correlation between the gut barrier regulation ability and protection against osteoporosis by probiotics was further verified using treatment with L. rhamnose (LGG) and L. plantarum (CCFM8610) strains with significant protective effects against TNF-α treatments in in vitro trials. These results are consistent with previous studies showing that oral LGG supplementation can increase bone formation in female mice by stimulating the growth of butyrate-producing gut bacteria, such as Clostridia, and attenuate tenofovir disoproxil fumarate-induced bone loss in male mice via a gut-microbiota-dependent anti-inflammatory mechanism [9,21]. Although the study of CCFM8610 alleviating osteoporosis was not reported, treatment with this strain has been demonstrated to play a significant role in gut damage caused by heavy metals and intestinal inflammation induced by DSS. Further results indicated that the underlying mechanism is associated with the recovery of tight junction proteins and inhibition of intestinal epithelial cell apoptosis [51,79]. In addition to the osteoporosis model, we evaluated the protection against alcoholic liver disease by L. casei and L. helveticus strains by determining tight junction proteins expression, liver pathological histopathology, oxidative stress level, liver function indices, and inflammatory cytokines in rats and confirmed a similar positive correlation between the intestinal barrier regulation ability and in vivo protection by probiotics (Fig. 6 and Fig. S5).
《4.4. A strong possibility: in vitro models of human cells to mimic/ predict the effect in vivo 》
4.4. A strong possibility: in vitro models of human cells to mimic/ predict the effect in vivo
A considerable number of studies have confirmed the protective effects of probiotics against metabolic diseases. However, there is a lack of an efficient method for screening these beneficial strains. Animal trials based on specific models have been recognized as reliable approaches for the simulation of metabolic dysfunction in humans. However, these models are deficient in efficiency and expense. It takes more than 30 days for the establishment of an ovariectomy-induced osteoporosis rat model and the experimental period for screening probiotics in a Lieber– DeCarli diet-treated mouse model to mimic chronic alcoholinduced liver injury always lasts for more than 50 days [62,80]. Compared with in vitro trials based on microbiological and cell biological techniques, the costs of animal feeding and surgery are relatively high. More importantly, animal studies are a serious ethical challenge, guiding principles that the three R’s, replacement, reduction, and refinement, were recognized as obligatory rules [81]. Correlation analysis is recognized as a common method to measure the degree of correlation between two or more variable factors. A number of studies used this method to evaluate the relationship between probiotics and its physiological function in vivo, such as the association of Bifidobacterium and urgency of defecation [82], probiotics-fermented massa medicata fermentata and intestinal homeostasis [83] and probiotic colonization and Clostridioides difficile infection [84]. To illustrate the correlation degree between the regulative ability on gut barrier in vitro and the alleviation of specific metabolic disease in vivo, we selected correlation analysis method. In the present study, the Caco-2 cell monolayer model proved to be a feasible in vitro method for the screening of probiotics against metabolic diseases. The Pearson correlation coefficient rXY is defined in statistics as the measurement of the strength of the relationship between two variables and their association with each other [85]. Pearson’s correlation coefficient returns a value between –1 and 1 and the absolute values of rXY ≥ 0.8 indicate a strong correlation between X and Y [86]. Similar to our study, a strong in vivo–in vitro correlation (rXY = 0.82) was found following the linear regression of the cumulative number of solvents permeated in vitro and the corresponding skin uptake in vivo measured with confocal Raman spectroscopy (CRS), suggesting that CRS is a powerful tool for profiling drug and vehicle delivery from dermal formulations [87]. According to the results of the present study, the Pearson’s coefficient correlation between cell and animal trials was 0.82 and –0.97, respectively, for osteoporosis and alcoholic liver disease (Figs. 3 and 7), indicating the reliability of the cell model to simulate the in vivo protective effects of the probiotics. Compared with animal models, the Caco-2 cell monolayer model has advantages in terms of the experimental period, cost, and throughput. Our results may provide a potential approach for the screening of probiotics against specific metabolic diseases.
《5. Conclusions》
5. Conclusions
In summary, this study demonstrated that Lactobacillus strains with excellent gut barrier regulation ability can protect against metabolic diseases. We developed an in vitro screening method based on a Caco-2 cell monolayer model for the identification of potential probiotics against specific metabolic diseases, such as osteoporosis and alcohol-induced liver disease.
《Acknowledgments》
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32122067 and 31820103010); the Natural Science Foundation of Jiangsu Province (BK20200084); and Collaborative innovation center of food safety and quality control in Jiangsu Province.
《Authors’ contributions》
Authors’ contributions
Yang Liu: methodology, software, formal analysis, writing original draft. Jiang Peng: visualization, methodology. Shiya Zhu: methodology, software. Leilei Yu: formal analysis, investigation. Fengwei Tian: formal analysis, investigation. Jianxin Zhao: validation, investigation. Hao Zhang: validation, investigation. Wei Chen: project administration, funding acquisition. Qixiao Zhai: conceptualization, writing review and editing, supervision, funding acquisition. All authors contributed to the article and approved the submitted version.
《Compliance with ethical guidelines》
Compliance with ethical guidelines
Yang Liu, Jiang Peng, Shiya Zhu, Leilei Yu, Fengwei Tian, Jianxin Zhao, Hao Zhang, Wei Chen, and Qixiao Zhai declare that they have no conflicts of interest or financial conflicts to disclose.
《Appendix A. Supplementary data》
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eng.2022.02.014.














 京公网安备 11010502051620号
京公网安备 11010502051620号




